Growth hormone
Generally about growth hormone[edit | edit source]
| Gland | adenohypofýza |
|---|---|
| Structure | 1-chain structure of 191 AMK |
| Receptor | growth factor receptors |
| Effects | increase in proteosynthesis, release of fatty acids from tissues, diabetogenic |
| OMG | 139250 |
Structure, synthesis[edit | edit source]
Growth hormone (somatotropin, somatotropic hormone, STH, growth hormone, GH) is a linear polypeptide of 191 amino acids with two internal disulfide bridges and M r 21,500. Of the growth hormones of other mammals, only monkey STH is close to it immunologically and chemically . It is also the only one that is biologically effective in humans, the others are completely ineffective. Somatotropin arises from a larger precursor of M r 28,000, the so-called pre-STH (pre-GH), which is also secreted into the blood, but has no physiological effect. STH is synthesized and secreted in adenohypophyseal somatotropes, which make up approximately 50% of the cells of the adenohypophysis and (along with lactotrophs) are among the acidophilic secretory cells of the anterior lobe of the pituitary gland, staining with acid dyes (e.g. eosin).
Receptor pro STH[edit | edit source]
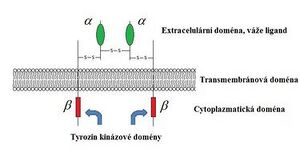
The growth hormone receptor is a protein of 620 amino acids with a large extracellular part, a transmembrane domain and a large part of the molecule in the cytoplasm, belonging to the family of cytokine receptors. STH has two receptor binding sites, i.e. when it binds to one receptor subunit, the other somatotropin binding site attracts the other receptor subunit. This creates a homodimer , which is necessary for the activation of the receptor and subsequently various intracellular cascades (primarily via the JAK2-Stat pathway of gene activation in the nucleus).
Transport STH[edit | edit source]
In plasma, STH binds to GHBP (GH binding protein), which is a large fragment of the extracellular domain of the receptor. GHBP is probably produced by cleavage of receptors for STH and its concentration is therefore proportional to the number of receptors for STH in tissues. About half of STH activity is bound, which creates a reserve of STH that can compensate for large fluctuations in its secretion. Free STH has a half-life in the blood of 20–50 min. Radioimmunoassay determines both the free and the bound form because the antibodies used have a higher affinity for the hormone than for the binding proteins.
Values[edit | edit source]
Endocrine regulation of growth
- healthy adults secrete approximately 40 µg of STH per day (18.6 nmol/day);
- adolescent young people secrete about 700 µg/day (32.5 nmol/day);
- the daily output of the hormone in adults was calculated to be 0.2–1.0 mg/day;
- in fasting adults, the morning concentration of STH in the blood is below 5 ng/ml (232 pm/l), sometimes even lower (2 ng/ml).
Genes[edit | edit source]
The gene for STH is located on the long arm of chromosome 17 in a cluster of five genes: STH-N encodes human STH, STH2 encodes a variant of STH produced in the placenta , CSH1 and CSH2 encode prolactin, and CSHP1 , which encodes a variant of the prolactin molecule. STH, which is the product of the STH-N gene, is present in the greatest amount and represents about 75% of STH in the blood.
Gene for hCS[edit | edit source]
The STH2 gene is mainly expressed in the placenta and its product is human chorionic somatomamotropin (hCS), which is also 191 amino acids long, but differs from "normal" STH by 13 amino acids. It is produced by the syncytiotrophoblast, it is found in large quantities in the maternal blood during pregnancy, but little passes into the blood of the fetus . In turn, it can reduce the secretion of STH from the pituitary gland in the mother. It is believed that hCS has a lactogenic effect (a positive effect on the growth of the mammary gland and on lactation) and most of the effects of growth hormone: it causes nitrogen, potassium and calcium retention, lipolysis and a decrease in glucose utilization in pregnancy, which helps the supply of glucose to the fetus . The amount of hCS produced is proportional to the size of the placenta.
Secretion of STH[edit | edit source]
Control of secretion by the hypothalamus[edit | edit source]
STH secretion is controlled by two hypothalamic hormones , which are secreted into the portal pituitary blood:
- GHRH (STH-RH, GRH, somatoliberin) – has a stimulating effect.
- Somatostatin (GHIH) – has an inhibitory effect.
Since neither GRH nor somatostatin can be directly measured, it is necessary to consider the change in STH secretion as a manifestation of the sum of both of these hypothalamic hormones. In addition to this regulation, a number of other factors are involved in the regulation of STH secretion (see below).
GHRH[edit | edit source]
GRH stimulates the synthesis and secretion of STH in the somatotropes of the adenohypophysis (by the formation of cAMP ). GRH of 40 and 44 amino acids is present in the human hypothalamus . GRH-secreting neurons are located in the nucleus arcuati and their axons terminate in the outer layer of the eminentia medialis . GRH is synthesized from a larger precursor of 107 or 108 amino acids (differs in the presence or absence of serine at position 103). Human GRH is highly similar to many gastrointestinal peptides, including secretin, gastrin, vasoactive intestinal peptide (VIP), and gastric inhibitory peptide. The half-life of GRH is 50 min. Administration of GRH leads to rapid secretion of STH (within minutes), peaks at 30 minutes and lasts for 60-120 minutes.
Somatostatin[edit | edit source]
Somatostatin inhibits the secretion of STH (reduces the formation of cAMP in somatotropes) and TSH (by increasing the direct inhibitory effect of thyroid hormones on adenohypophyseal thyrotropes). Cells secreting somatostatin are located in the periventricular region of the hypothalamus immediately above the optic chiasm, and their nerve endings are diffuse in the outer layer of the eminentia medialis. Somatostatin is a tetradecapeptide and, in addition to the hypothalamus, it is also found in the D-cells of the pancreatic islets of Langerhans , in the gastrointestinal mucosa, and in the parafollicular cells (C-cells) of the thyroid gland. The somatostatin precursor has 116 amino acids. Somatostatin-14 is the main one in the hypothalamus, somatostatin-28 is in the intestine. Somatostatin also has strong inhibitory effects on other hormones, including insulin and glucagon (paracrine in the islets of Langerhans), gastrin, secretin, and VIP (in the gastrointestinal mucosa). Somatostatin secretion increases with elevated STH and IGF-I levels (see below). A long-acting synthetic analogue of somatostatin (octreotide acetate) is used in therapy (for conditions of STH excess).
Shock secretion[edit | edit source]
STH is released into the blood in pulses. This irregular, intermittent secretion is associated with sleep and depends on age. It reaches its peak 1-4 hours after falling asleep (during sleep stages 3 and 4). These nocturnal secretion pulses amount to about 70% of the total amount secreted per day and are higher in children, decreasing with age. GRH secretion is episodic and its increase correlates with an increase in STH secretion. Somatostatin secretion is rather tonic.
Insulin-like growth factors[edit | edit source]
The effect of growth hormone is mainly mediated by insulin- like growth factors (IGF-I and IGF-II) (but STH also has some direct effects - via cAMP). IGF-I (originally somatomedin C) and IGF-II (originally somatomedin A) are the main and, in humans, probably the only somatomedins in the blood. Both have a structure similar to the proinsulin molecule and both bind to plasma proteins (which prolongs their half-life in the blood). To date, six IGF-binding proteins (IGFBPs) have been identified, which are differently distributed in different tissues. All are present in plasma. About 95% of IGF circulates in the blood bound to IGFBP-3, whose serum level is directly proportional to the concentration of STH and the nutritional status of the organism. STH deficiency causes lower concentrations of both IGF-I and IGF-II, but excess STH increases IGF-I without affecting IGF-II.
IGF-I[edit | edit source]
The single gene for prepro-IGF-I is located on the long arm of the chromosome12. IGF-I is a basic polypeptide of 70 amino acids. Its receptor (type I receptor) is located on the cell membrane and resembles the insulin receptor (two alpha and two beta chains). Binding of IGF-I to type I receptors leads to stimulation of tyrosine kinase and subsequent autophosphorylation of tyrosine molecules, which leads to cell differentiation or division (or both). IGF-I receptors are "down-regulated" at elevated IGF-I concentrations. Conversely, reduced IGF-I concentrations lead to an increase in IGF-I receptors. The most important site for IGF-I synthesis is the liver (but it is also produced by other organs, e.g. cartilage). It seems that it is transmitted to neighboring cells, which it affects by paracrine action (or is an autocrine factor that affects the cell from which it originates), so it may not have the greatest effects in the blood.
- Effects of IGF-I
- increases the incorporation of sulfate (SO 4 ) 2- into the cartilages and thus stimulates their growth ,
- stimulating effect on hematopoiesis ,
- stimulating effect on the formation of ovarian steroids, proliferation and differentiation of myoblasts,
- differentiation of the eye lens.
- suppresses STH secretion and stimulates somatostatin secretion.
Before birth, the secretion of IGF-I does not depend on growth hormone (see below), but after birth, STH is stimulated and IGF-I has a significant stimulating effect on growth. The concentration of IGF-I in the blood rises in childhood and peaks at the time of puberty, then decreases to low values in old age. IGF-I is currently produced by recombinant DNA technology. Administration of IGF-I increases nitrogen retention and decreases blood urea nitrogen and stimulates growth in STH-resistant patients (Laron dwarfism, see below).
IGF-II[edit | edit source]
IGF-II is a neutral peptide consisting of 67 amino acids. The gene for prepro-IGF-II is located on the short arm of chromosome 11 (near the gene for preproinsulin) and in adults it is expressed only in the choroid plexus and in the meninges. The type II receptor preferentially binds IGF-II and is identical to the mannose-6-phosphate receptor, a single-chain transmembrane protein. Most of the effects of IGF-II appear to be mediated by interaction with the type I receptor, and independent effects of IGF-II through the type II receptor have also been described. IGF-II is largely independent of STH and is important in fetal growth before birth.
Other factors regulating STH secretion[edit | edit source]
In addition to GRH and somatostatin, other factors regulate STH secretion:
- Stimulating
- hypoglycemia (e.g. administration of 2-deoxyglucose, which causes an intracellular glucose deficit, increases STH secretion),
- intake of food with a high proportion of proteins, but also protein-caloric malnutrition (leads to a decrease in IGF-I formation and thus to the elimination of negative feedback),
- intravenous infusion of amino acids (e.g. arginine), starvation (this will mobilize fat as an energy source and conserve protein),
- physical exertion, stress, dopaminergic and α-adrenergic agonists.
Sex hormones, especially estrogens, and ß-adrenergic antagonists increase STH responses to stimuli (such as arginine, insulin).
- Lowering level
Oral or intravenous administration of glucose reduces the level of STH (used in the diagnostic test for acromegaly). Fatty acids suppress STH responses to some secretory stimuli, such as arginine and hypoglycemia. Also, excess cortisol and hypo- and hyperthyroidism reduce the growth hormone response to secretory stimuli. STH secretion is inhibited by ß-adrenergic agonists and dopaminergic antagonists.
Effects of growth hormone[edit | edit source]
The main effect of STH is the stimulation of linear growth (most of the growth effect is mediated by IGF -I):
- STH increases proteosynthesis through IGF-I by increasing amino acid uptake and directly increasing mRNA transcription and translation .
- STH reduces proteocatabolism by mobilizing fat (as a more efficient energy source). It directly causes the release of fatty acids from adipose tissue (thereby promoting ketogenesis) and enhances their conversion to acetyl-CO.
- STH also affects carbohydrate metabolism . It is diabetogenic because it increases the release of glycogen from the liver and has an anti-insulin effect in the muscles.
- Insulin resistance
Its excess reduces the utilization of carbohydrates (inhibits glucose phosphorylation) and disrupts glucose uptake in cells. This insulin resistance is probably caused by a post-receptor disorder of insulin action. STH thus worsens clinical diabetes, and in case of overproduction of growth hormone (adenohypophysis tumor secreting STH), 25% of patients will develop diabetes. Growth hormone does not directly stimulate insulin secretion , but the hyperglycemia it induces secondarily stimulates the pancreas and can eventually lead to B-cell exhaustion (reduced insulin production). Growth hormone causes a positive balance of nitrogen and phosphorus. Increases plasma phosphate levels and decreases blood urea nitrogen and amino acid levels. Independent of the adrenal glands ( aldosterone) the excretion of Na + and K + is reduced (these electrolytes are directed to growing tissues). Furthermore, resorption of Ca 2+ in the digestive system and excretion of 4-hydroxyproline increases (due to increased turnover of collagen ).
Growth and growth hormone[edit | edit source]
The role of growth hormone in prenatal growth is probably only marginal (although fetal plasma STH values are high). During intrauterine life, fetal growth is thought to be controlled through IGF-I. Fetal IGF-I is regulated through placental glucose transport , which also controls fetal insulin release . The glucose -insulin-IGF-I axis is thus primarily used in prenatal growth. Postnatal growth is regulated by the growth hormone-IGF-I axis, and therefore IGF-I levels rise after birth. At birth, STH concentrations are elevated, but IGF-I concentrations are relatively low in neonates. Afterwards, resting levels of STH decrease, but pulses of STH secretion appear, especially at puberty (sex steroids increase the amplitude of STH secretion pulses). This increases IGF-I levels during childhood , peaking at 13–17 years of age . However, IGF-II levels do not change during postnatal life.
Prenatally[edit | edit source]
The system of IGF-I and II and its binding proteins is one of the endocrine and paracrine growth systems that regulate fetal and placental growth. The levels of IGFs and IGFBPs in the fetus are significantly influenced by the level of nutrition. In malnutrition, IGF-I and IGFBP-3 levels decrease and IGFBP-2 levels increase. Children with a mutation of the IGF-I gene are born with low birth weight, suffer from postnatal growth disorder, mental retardation and insulin resistance . During intrauterine life, IGF-I levels gradually increase in the growing fetus from the 18th to the 40th week of gestation . In children who were born with low birth weight, there is already a significantly lower level of IGF-I and IGFBP-3 in the umbilical cord blood compared to children with a weight appropriate for age (while the levels of IGFBP-1 are significantly higher in these children).
For a number of diseases in adulthood, which include hypertension, ischemic heart disease or type 2 diabetes mellitus , it is assumed that their origin, course and prognosis depend on the joint action of environmental factors and genetic predispositions. Among the risk factors is intrauterine fetal growth disorder, which subsequently leads to lower birth weight and length of the child at birth.
IUGR[edit | edit source]
Intrauterine growth retardation (IUGR) and low birth weight and length for gestational age (SGA) occur in approximately 3% of children. Intrauterine growth is influenced by a number of fetal, maternal, placental and demographic factors. The IGF-I, IGF-II and their binding protein (IGFBP) system, as well as some polymorphisms in the IGF-I gene promoter, seem to be a suitable candidate system for the link between intrauterine growth disorder and some diseases in adulthood.
Postnatally[edit | edit source]
In young people, in whom the growth fissures are not yet fused with the diaphyses of the long bones, growth can be stimulated by growth hormone or, conversely, inhibited by its lack. Prolonged exposure causes gigantism . Once the epiphyseal fissures close, growth in length is no longer possible. If STH is then in excess, it causes characteristic bone and soft tissue deformities, referred to as acromegaly .
Excess STH[edit | edit source]
Tumors from the somatotropic cells of the adenohypophysis release large quantities of STH, which leads to:
- in children to gigantism ,
- in adults to acromegaly .
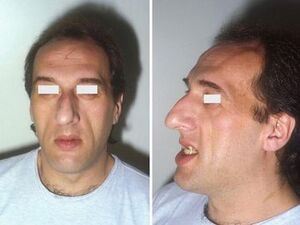
Acromegaly[edit | edit source]
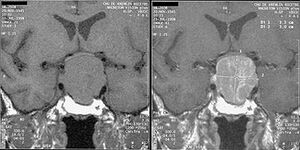
In patients affected by acromegaly, STH hypersecretion is also accompanied by prolactin hypersecretion in 20-40% . Acromegaly can be caused by both intrapituitary and extrapituitary tumors secreting growth hormone, as well as hypothalamic tumors secreting GRH (these are rare, however). The main findings in acromegaly are manifestations of local effects of the tumor (enlargement of the Turkish saddle, headaches , visual disturbances) and also manifestations of STH excess:
- enlarged legs and hands, protrusion of the lower jaw ( prognathia );
- excessive growth of the facial, frontal and basal bones of the facial part of the skull and thus a coarse facial expression ( acromegalic facies );
- body hair increases (hirsutism);
- skeletal changes and a tendency to osteoarthritis ;
- lactation even without pregnancy (in 4%).
Gigantism[edit | edit source]
Pituitary gigantism is characterized by excessive linear growth . The growth rate tends to be excessively high, and other somatic symptoms typical of acromegaly may also be present in addition to a taller stature.
Lack of STH[edit | edit source]
The incidence of STH deficiency is estimated to be 1:10,000 worldwide.
Pituitary disorders[edit | edit source]
Most patients with growth hormone deficiency appear to lack GRH. Some of them have a sufficient number of adenohypophyseal somatotropes with significant reserves of STH. Long-term treatment with GRH in these patients can lead to STH release and growth improvement. Patients with pituitary tumors or rare cases with congenital absence of the pituitary gland do not have somatotropes. In the past, families have also been described that lack various parts of the STH gene responsible for the formation of STH. These individuals initially respond to the administration of exogenous STH, but many of them soon develop high levels of antibodies that terminate the beneficial course of therapy.
Disorder of receptors for STH[edit | edit source]
In another group of nanic patients, STH plasma levels are normal or elevated, but STH receptors are inactive (or completely absent) due to a loss-of-function receptor gene mutation. The resulting condition is referred to as growth hormone insensitivity – Laron's dwarfism , which is characterized by low plasma concentrations of IGF-I. After administration of exogenous STH, IGF-I levels do not increase, but administration of IGF-I increases growth rate and suppresses STH concentrations. The plasma level of IGFBP-3 is also significantly reduced. This disorder is inherited in an autosomal recessive manner .
- Defect in IGF-I production
Similarly, African pygmies have normal plasma concentrations of STH, low levels of IFG-I, and normal concentrations of IGF-II. They do not respond to exogenous STH by improving growth rate and increasing IGF-I levels because they have a congenital inability to produce IGF-I, which is more important for growth stimulation than IGF-II. The pubertal growth spurt (sudden growth spurt) is absent in pygmy children, suggesting that IGF-I is necessary to achieve a normal peak growth rate. Presumably, treatment with IGF-I would accelerate growth in this population during childhood and puberty, but there are no reports of such treatment to date.
Clinical manifestations[edit | edit source]
Congenital deficiency[edit | edit source]
- normal body length at birth;
- growth rate slows soon after birth (can be detected by careful measurements during the first year);
- patients are short, obese with an immature facial expression, high-pitched voice, and delayed bone maturation;
- hypoglycemia and convulsions may be present in newborns or children , boys may have a micropenis.
Intelligence is normal in patients with STH deficiency, unless brain development has been disrupted by repeated or severe hypoglycemia . Anatomical defects in the midline may also appear: optic hypoplasia with visual disturbances from nystagmus to blindness combined with various hypothalamic disorders (including diabetes insipidus), septum pellucidum is missing in about half of patients (on CT or MRI ), cleft palate affects about 7 % of patients. Milder forms of partial STH deficiency are also described. There are several types of congenital STH deficiency:
- type IA is inherited in an autosomal recessive manner, patients have a defect in the STH gene, some children have a short birth length;
- type IB is also inherited autosomal recessively, but there is no gene depletion;
- type II is inherited in an autosomal dominant manner ;
- Type III affected patients suffer from X-chromosome-linked STH deficiency.
Acquired deficiency[edit | edit source]
If STH deficiency appears in late childhood or adolescence, it is an acquired STH deficiency, the cause of which can be a craniopharyngioma, germinoma, glioma, etc. If other pituitary hormone deficiency symptoms also appear, it can also be a hypothalamo-pituitary tumor. Also, empty sella syndrome (hypothalamo-pituitary abnormalities) can be associated with STH deficiency, more often in childhood than in adulthood. Irradiation of the head in the hypothalamo-pituitary region in the treatment of head tumors can lead to growth hormone deficiency in 6-24 months as a result of damage to the hypothalamus or pituitary gland. It is necessary to carefully monitor these patients for growth disorders after radiation.
Diagnosis of STH concentration[edit | edit source]
Basal STH values are low in healthy children and in patients with STH deficiency. Therefore, STH deficiency is diagnosed by insufficient rise of STH in the serum after provocation stimulation. Tests may not reveal STH deficiency if the STH response to stimulation is normal.
With the exception of sleep tests, the tests should be performed after an overnight fast (because e.g. carbohydrate intake suppresses the STH response, see above). STH secretion is also inhibited in obesity, and therefore "stronger" children may appear to be deficient in STH.
Serum STH levels should rise during stage 3 and 4 sleep (about 90 minutes after falling asleep) or 10 minutes after vigorous exercise. After an overnight fast, STH levels should also rise after arginine infusion or orally administered levodopa (a dopamine agonist). STH levels also rise in acute hypoglycemia after insulin administration . However, this insulin tolerance test carries the risk of convulsions if there is an excessive drop in glucose levels. The patient must therefore be under the supervision of a doctor, must not have a history of hypoglycemic seizures, and must have a normal glucose level at the beginning of the test. A 50% drop in blood glucose occurs within 20-40 minutes, which should be followed by a rise in serum STH (and cortisol). Glycemia should be monitored continuouslyand have an intravenous line ready for the infusion of a 10-25% glucose solution in case the patient loses consciousness and falls into hypoglycemic convulsions (after the infusion, however, the blood glucose must not exceed the range too high, otherwise there would be a risk of hyperosmolality! ) .
Treatment in the Czech Republic[edit | edit source]
Since 1992, in the Czech Republic, treatment with growth hormone has been fully covered by health insurance (the treatment of one pituitary tumor in our country costs around 250,000-400,000 per year). The indication for the treatment of STH, which is produced by recombinant DNA technology (see below), is congenital or acquired growth hormone deficiency (see above), Turner syndrome , since 1995 growth failure due to chronic renal insufficiency (there is a certain resistance to action of STH and IGF-I), since 2001 Prader-Willi syndrome and since 2003, postnatal growth failure following intrauterine growth retardation (the newborn has a low birth weight or length, in 10-15% of such affected children there is no postnatal growth acceleration due to reduced growth hormone production or reduced sensitivity to STH). In severe growth hormone deficiency, due to its metabolic effects, it continues to be administered in a small dose even after the end of growth in adolescence and adulthood. As of 2020, it is also indicated for RASopathies such as Noonan syndrome.
Children are treated in 12 pediatric centers in the Czech Republic. Children with chronic renal insufficiency are treated in centers where they are simultaneously treated with dialysis. The growth hormone is applied daily before going to bed (to imitate the natural rhythm) into the subcutaneous tissue using application pens that ensure its precise dosage. Treated children are regularly monitored for possible adverse effects of therapy – impaired glucose tolerance, sodium and water retention, epiphysiolysis of the femoral head is described in isolated cases, and recurrence of lymphedema in Turner syndrome .
Recombinant DNA technology[edit | edit source]
Until 1986, the only classical method to treat STH deficiency was replacement therapy with human STH (hSTH) from deceased donors. In 1985 and later, they were diagnosed with Creutzfeldt–Jakob disease , a degenerative neurological disease (otherwise rare in young patients) that developed within 10–15 years of receiving natural hSTH. Because it was possible that the pituitary glands of the human donors were contaminated with prionsand after transmission to STH-deficient patients caused their death, natural growth hormone was withdrawn from circulation from all sources. Growth hormone is now obtained by recombinant DNA technology. Commercial growth hormone commonly available today has 191 amino acids in the natural sequence (somatropin) and 192 amino acids in the methionyl form (somatrem). GRH was first isolated from a pancreatic tumor stimulating the secretion of STH (GRH from pancreatic tumors has the same structure as hypothalamic GRH). IGF-I is also now produced by recombinant DNA technology.
Conclusion[edit | edit source]
The level of growth hormone must be constantly regulated. Both high and low concentrations of STH cause serious health disorders. Growth hormone therapy has a positive effect in patients who are indicated for this treatment. It is important to start the treatment quickly, which brings a chance for a better overall effect of the therapy. Early detection of a growth disorder in a child, establishing the correct diagnosis and early growth hormone therapy fundamentally affect the overall health status and final height of child patients.
Links[edit | edit source]
Related articles[edit | edit source]
External links[edit | edit source]
Source[edit | edit source]
With the permission of the author Klára Mědílková; translated from WikiSkripta
References[edit | edit source]
- GREENSPAN, F. S – BAXTER, J.D. Základní a klinická endokrinologie. 1. edition. H+H, 2003. ISBN 80-86022-56-0.
- GANONG, William F. Přehled lékařské fyziologie. 20. edition. Galén, 2005. ISBN 80-7262-311-7.
- TROJAN, Stanislav. Přehled lékařské fyziologie. 4. edition. Grada, 2003. ISBN 80-247-0512-5.
- BLAHOŠ, J – BLEHA, O. Endokrinologie. 1. edition. 1979.
- KYTNAROVÁ, J – ZLATOHLÁVKOVÁ, B – FEDOROVÁ, M. Intrauterinní růstová retardace a fetální původ chorob v dospělosti. Česko-slovenská pediatrie. 2008, y. 63, no. 6, p. 320-326, ISSN 1803-6597.
- POMAHAČOVÁ, R. Léčba růstovým hormonem v dětském věku. Farmakoterapie. 2007, y. 6, no. 5, p. 501-506, ISSN 1803-6597.