Catecholamines
Catecholamines are hormones produced by chromaffin cells of the medulla adrenal gland (gl. suprarenalis) which is actually a special sympathetic ganglion. They are also formed in ``adrenergic sympathetic postganglionic neurons or in the ``CNS and act as neurotransmitters. They are derived from tyrosine. Their name comes from the English catechol, i.e. pyrocatechol (i.e. benzene-1,2-diol).
Among the catecholamines, produced in the neurons and adrenal medulla, are
- adrenalin (epinephrine, from the Greek "epi" nad, "nephros" kidney),
- noradrenaline (norepinephrine) and dopamine. Derivatives include, for example, isoprenaline.
Synthesis of catecholamines[edit | edit source]
The synthesis is induced by various stressful situations, when the adrenergic nuclei of the hypothalamus start sending out impulses. These impulses lead to the secretion of acetylcholineu by the preganglionic neurons that innervate the adrenal medulla[1].
- The initial substrate is tyrosine, which is hydroxylated in the presence of tetrahydrobiopterin and molecular oxygen by the enzyme tyrosine hydoxylase' to Template:L-DOPA' (3, 4-dihydroxyphenylalanine).
- This is followed by decarboxylation to dopamine by the enzyme DOPA-decarboxylase' with the coenzyme pyridoxal phosphate, present in all tissues synthesizing catecholamines. Dopamine is hydroxylated in the adrenal medulla and in ``noradrenergic neurons by ``dopamine-β-hydroxylase to form noradrenaline. This reaction requires ascorbic acid, which acts as an oxygen-carrying coenzyme.
- Finally, adrenaline is produced in the medulla of the adrenal glands by methylation of noradrenaline with the help of phenylethanolamine-N-methyltransferase' (PNMT). The donor of the methyl group in the last reaction is S-adenosylmethionine (SAM), which is converted to S-adenosylhomocysteine by cleavage of the methyl.
The enzyme equipment of a certain cell (neuron or chromaffin cell of the adrenal medulla) is decisive for the production of the resulting catecholamine. However, it can be said that dopamine or norepinephrine is produced in neurons', while the main product of the adrenal medulla is adrenaline. Only the adrenal medulla contains all 4 enzymes necessary for the production of all catecholamines.
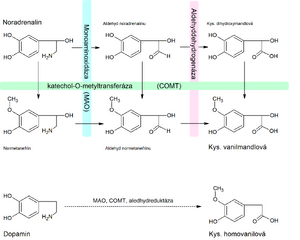
Degradation[edit | edit source]
2 enzymes are important for the degradation of catecholamines - monoamine oxidase (MAO)' and catechol-O-methyltransferase (COMT). The action of COMT induces methylation of the hydroxyl group on the benzene nucleus. Similarly to the synthesis of adrenaline, the methyl donor is S-adenosylmethionine (SAM). The action of MAO results in oxidation of the amino group to an aldehyde. An aldehyde is oxidized to a carboxylic acid, or it can be reduced to an alcohol.
The end product of the degradation of adrenaline and noradrenaline is Vanillamandelic acid''. The end product of dopamine degradation is homovanillic acid. Degradation products of catecholamine metabolism are excreted in urine.
MAO and COMT inhibitors slow down the breakdown of catecholamines and thus increase their effects. Medically, some MAO inhibitors are used as antidepressants. COMT inhibitors are used as antiparkinson drugs because they increase the availability of dopamine.
Effects[edit | edit source]
Catecholamines act in target cells through binding to membrane G-protein coupled receptors. The resulting effect depends on which G-protein the receptor is coupled to.
Adrenaline and noradrenaline bind to the so-called adrenergic receptors. There are α- and β-adrenergic receptors. Both types of receptors can be further divided into several subtypes: α1, α2, β1, β2, β3. α1 receptors are coupled to the Gq protein, which activates phospholipase C and as a result the intracellular concentration of Ca2+ will increase.α2 receptors are coupled to Gi by a protein that inhibits adenylate cyclase and the intracellular concentration of cAMP will decrease. β-adrenergic receptors' activate adenylate cyclase via the Gs protein and the intracellular cAMP concentration increases. Changes in cAMP and Ca2+ concentrations determine the resulting biological effect. Both epinephrine and noradrenaline act on both types of receptors, but noradrenaline has a higher affinity for α-adrenergic receptors, while epinephrine has a higher affinity for β-adrenergic receptors.
Dopamine binds to dopamine receptors, which are closely related to adrenergic receptors. There are known 5 types of dopamine receptors' (D1–D5). D1 and D5 receptors increase cAMP levels, while D2, D3 receptors and D4 reduce the level of cAMP.
Meaning[edit | edit source]
Catecholamines are mediators of the sympathetic part of the vegetative system. They ensure the connection between the neuron and the effector tissue.
Influencing their production, leaching and degradation is a very attractive topic from the point of view of pharmacology. Today, a large number of substances are known that influence the processes associated with catecholamines. Because the administration of catecholamines manifests itself in various sympathetic reactions, we divide the drugs into:
- sympatholytics - they weaken the manifestations of the sympathetic,
- sympathomimetics - they strengthen the manifestations of the sympathetic.
Treatment with these substances is an important step in, for example, shock conditions, heart failure, hypertension and asthma.
Links[edit | edit source]
Related Articles[edit | edit source]
External links[edit | edit source]
Source[edit | edit source]
- KOOLMAN, Jan – RÖHM, Klaus-Heinrich. Color Atlas of Biochemistry. 1. edition. Prague : Grada, 2012. 512 pp. ISBN 978-80-247-2977-0.
- MATOUŠ, Bohuslav, et al. Basics of medical chemistry and biochemistry. 1. edition. Prague : Galen, 2010. 540 pp. ISBN 978-80-7262-702-8.
- WEIL, P.Anthony. The diversity of endocrine system : Catecholamines & thyroid hormones are made from tyrosine. In Harper's illustrated biochemistry. 28. edition. New York : McGraw-Hill Companies, 2009. 693 pp. pp. 435-436. ISBN 978-0-07-163827-2
- GANONG, William F. Review of Medical Physiology. 20. edition. Prague : Galen, 2005. 890 pp. ISBN 80-7262-311-7.
References[edit | edit source]
- ↑ SMITH, Colleen – MARKS, Allan D – LIEBERMAN, Michael. Mark's basic medical biochemistry : A clinical approach. 2. edition. Maryland : Lippincott Williams & Wilkins, 2005. 977 pp. Chapter 43. pp. 791. ISBN 0-7817-2145-8.