Plasma and urine proteins
Plasma proteins[edit | edit source]
The proteins in blood serum or plasma are many and are produced by various cells. The biosynthesis of the vast majority of plasma proteins takes place in the liver. A smaller part is synthesized in other cells: e.g. lymphocyte (immunoglobulins) and enterocytes (e.g. apoprotein B-48). Protein degradation takes place inhepatocytes and the mononuclear phagocytic system, where proteins are degraded predominantly after complex formation (e.g. antigen-antibody complex and hemoglobin-haptoglobin complex). Intracellularly, peptide bonds of proteins are hydrolyzed by proteases and peptidases to form amino acids. Another way serum proteins are removed is via excretion, which is facilitated by the kidneys and the gastrointestinal tract.
The total serum concentration of proteins is 65-85 g/L. Because plasma proteins are osmotically active, their physiological concentration contributes to a colloid osmotic pressure (oncotic pressure) of 3.33 to 3.52 kPa (25 to 26.4 torr). The concentration of proteins in plasma is slightly higher than in serum because plasma contains coagulation factors.[1]
Functions of plasma proteins[edit | edit source]
Plasma proteins are necessary for a variety of blood/plasma functions:
- Maintenance of oncotic pressure
- Transport of lipophilic compounds, such as some hormones (thyroid hormone bound to transthyretin), vitamins, lipids (bound to albumin), bilirubin bound to albumin) and drugs.
- Nutrition function
- Maintaining acid-base balance
- Hemocoagulation a fibrinolysis
- Humoral immunity
- Specific immunity (immunoglobulins)
- Non-specific immunity (complement, acute-phase proteins)
Overview of plasma proteins[edit | edit source]
|
Albumin[edit | edit source]
Albumin is the most common serum protein, it accounts for approximately 55 to 65% of total serum proteins (average blood concentration is 40 g/L[2]). It is synthesized in the liver and its production depends on amino acid intake.
- Albumin is crucial for the maintenance of the oncotic pressure of the plasma. Decreased albumin concentrations (hypoalbuminemia) below 20 g/L usually lead to edema.
- It acts as a carrier of various substances, enabling the transport of bilirubin, heme, steroid compounds, thyroxine, fatty acids, bile acids, metals, some drugs, among others.
- Albumin acts as a protein reserve, serving as a source of amino acids. During malnutrition, its concentration decreases; however, serum albumin levels are not a good indicator of early protein malnutrition because albumin has a long half-life and a large reserve. For this reason, albumin is a better long-term marker of nutrition.
Synthesis of albumin[edit | edit source]
The synthesis of albumin involves multiple steps. Preproalbumin, a precursor of albumin, is synthesized by hepatocytes within the cytoplasm. Subsequently, preproalbumin enters the endoplasmic reticulum, where it is transformed to proalbumin: the most abundant intracellular form of albumin. Next, proalbumin enters the Golgi apparatus, where it is transformed to albumin. It is then excreted out of the cell.[3]
Acute-phase reactants/proteins[edit | edit source]
The acute phase reaction is a physiological process that manifests itself in the systemic release of inflammatory mediators due to the development of pathologic processes (inflammation, trauma, surgeries, myocardial infarction, tumors, childbirth, excessive exercise, etc...). All the just mentioned situations can induce the increase of the concentration of certain proteins (positive acute-phase reactants) or their decrease (negative acute-phase reactants). However, the specific proteins involved and their degree of involvement will vary based on the underlying cause (e.g., bacterial infection will increase different proteins than trauma).
These mediators serve to establish an appropriate response to a certain pathologic process and mutual communication and regulation of ongoing events. Additionally, these proteins produce the general symptoms associated with the inflammatory response (fever, muscle, and joint pain). Substances whose synthesis arises as a result of a known pathology or when their concentration corresponds to the degree of tissue damage are of clinical importance. Such substances are called markers. By determining their presence and concentration, diagnoses can be confirmed, tissue damage extent can be evaluated, and therapy course can be monitored.
Positive acute-phase reactants (APP)[edit | edit source]
They can be divided into the following groups based on their effect or purpose:
Immune response proteins[edit | edit source]
- The purpose of some APP is to neutralize foreign substances (including microorganisms) that cause inflammation. Examples may be:
- C-reactive protein (CRP)
- Complement cascade (all are increased, but since C3 and C4 have the highest physiological plasma concentrations, their increase is the most pronounced)
- Tumor necrosis factor α (TNF-α), interleukin 1 (IL-1) and interleukin 6 (IL-6)
Proteins that prevent the collateral damage caused by the inflammatory response[edit | edit source]
- During inflammation, immune cells (such as phagocytes) release cytotoxic compounds that may damage not only the pathogen but also healthy tissues, which would cause undesirable side effects. To avoid this damage, during the inflammatory response, the organism produces proteins that inactivate proteolytic enzymes and reactive oxygen species (ROS) mitigating the damage to its tissues. Such compounds include:
Protease inhibitors
Proteins that decrease the synthesis of ROS - not only ROS scavengers but also proteins that bind transition metals (mostly iron and copper) whose presence may worsen the inflammatory process. As a result, decreased synthesis of ROS (via Fenton reaction) occurs. Such proteins include:
Proteins whose purpose is the transport of waste away from the source of inflammation
- Coagulation factors and proteins that induce tissue regeneration such as fibrinogen
The purpose of some positive reactants, such as procalcitonin (PCT) remains unknown. Despite the unknown purpose, its increase of plasma concentration may be of great importance when determining the nature of acute-phase reaction. Therefore, even some substances with unknown physiological functions may be clinically useful.
The rate of increase of acute-phase proteins[edit | edit source]
The rate of increase of acute-phase proteins varies considerably. Therefore, for clinical purposes, we can divide acute-phase proteins into three groups: early, intermediate, and late APP based on the rate of increase of their plasma concentrations.
Early positive acute-phase proteins[edit | edit source]
These are proteins with a very short biological half-life. Changes in their plasma concentrations are evident as early as 6-10 hours after the onset of the pathology. The rise usually peaks during the second and third days. The main representatives are CRP and SAA. More recently, PCT is used in clinical practice
- C-reactive protein
C-reactive protein (CRP) is one of the most important acute-phase proteins in diagnostics. This protein primarily functions in opsonization - it forms insoluble complexes (precipitates) with C-polysaccharide of pneumococci (thus earning the name C-reactive protein).[4]
Physiologically, the plasma concentration of CRP should not exceed 8 mg/L.[5] Acute bacterial infections (and rarely mycotic infections) cause a quick and sharp increase of CRP (usually above 60 mg/L, typically up to 200 mg/L, higher concentrations reveal a higher extent of infection). On the other hand, a viral infection usually leads to a minor increase of CRP in plasma (usually below 40 mg/L, sometimes the CRP values remain unchanged).[6] Plasma concentration of CRP increases as early as 4 hours after the beginning of the acute-phase reaction. Moreover, within the first two days, its concentration can increase to more than 1000-fold its physiological concentration. Peak concentration is reached between 24 and 48 hours. The determination of plasma concentration of CRP, therefore, is helpful in early decision with antibiotic therapy.[4] Additionally, the biological half-life of C-reactive protein is approximately 24 hours; therefore, CRP concentrations in plasma closely reflect the course of the acute-phase reaction.[7] If antibiotic therapy is successful, CRP plasma levels will quickly decrease. Otherwise, CRP levels will remain high or keep increasing.
Plasma concentrations of CRP can be used to identify the beginning post-surgery infections. Although CRP may be elevated after the surgery even without any infection, the third day after the infection, the CRP values should return to normalcy. If the return of CRP to its physiological values is slow (or absent), an ongoing infection may be present.
A mild increase of CRP may be observed in myocardial infarction. Generally speaking, mildly elevated CRP values (usually up to 10 mg/L) may be a marker of increased cardiovascular risk.[8] CRP values may be increased in autoimmune diseases and may be used in their long-term monitoring.[9]
The main disadvantage of CRP evaluation is its relatively low specificity. Additionally, CRP does not reflect the magnitude of the acute-phase reaction as well as procalcitonin does. These two markers are, to an extent, complementary.
Recently, procalcitonin (PCT) has reached a major interest in clinical practice. This 116-amino-acid-long protein (13,000 g/mol) is synthesized by the C cells of the thyroid gland as a precursor of calcitonin. During inflammation (e.g., generalized bacterial inflammation), this molecule can be synthesized by other cells throughout the body, especially by neuroendocrine cells of the lungs and intestine, but also in other organs [10]. As a result, during a bacterial infection, the plasma concentration of this protein increases. Procalcitonin synthesized during infection or sepsis is not converted into calcitonin.[11] The exact pathophysiological role of procalcitonin remains unknown, but it has been speculated that it can play a role in inflammation regulation and that it has an analgesic effect. The half-life of procalcitonin is 1 day and its concentration in plasma rises up to 20-fold of its physiological values as soon as 2 to 3 hours after the initiation of the acute-phase reaction. The increase of procalcitonin concentrations can be seen only in generalized bacterial, mycotic, or protozoal infections, but not during viral infections. A minor increase of PCT in plasma can be seen after polytrauma, burn patients, or after major surgeries of the abdomen.
Assessment of PCT[edit | edit source]
PCT can be assessed using highly sensitive PCT-LIA (Luminescence Immunoassay). This method uses two monoclonal antibodies, one of which has an affinity towards the C-terminal sequence of procalcitonin, and the other has an affinity towards the central part of the procalcitonin. Antibodies against the C-terminal sequence are immobilized on the surface of the test tube, while the antibodies against the central part of procalcitonin are soluble and then these are traced with a fluorescent dye. This method requires a luminometer; and approximately 20 μL of serum or plasma.
A quick method for the assessment of PCT is immunochromatography for procalcitonin (PCT-Q). This method requires 200 μL of serum or plasma and the result is available within 30 minutes. This test is recommended for quick diagnostics of acute pancreatitis.
PCT values[edit | edit source]
Normal values (ng/mL) <0.5; chronic inflammatory processes <0.5–1; bacterial infection complicated by systemic reaction 2–10; SIRS 5–20; severe bacterial infections - sepsis, MODS 10–1000. Elevated PCT levels persist during prolonged sepsis, while levels of some other cytokines decrease.[11]
[edit | edit source]
PCT can be elevated after surgeries, multiple traumas, heat-induced tissue damage, or cardiogenic shock. Additionally, elevated PCT can be seen in neonates within the first 48 hours past birth.[11]
The comparison of PCT, CRP, IL-6, and WBC shows (all of which can be used as markers of acute inflammation) that PCT is both the most sensitive and selective marker to diagnose and distinguish bacterial and non-bacterial infections.[12]
Intermediate positive acute-phase proteins[edit | edit source]
These are proteins whose concentrations increase 12 to 36 hours after the beginning of the acute-phase reaction and peak at the end of the first week. This group encompasses orosomucoid, α1-antitrypsin, haptoglobin, and fibrinogen.
Late positive acute-phase proteins[edit | edit source]
These encompass the complement proteins C3 and C4, and ceruloplasmin, among others. Their plasma concentration increase 48 to 72 hours after the beginning of the acute-phase reaction and peak 6 to 7 days afterward. These markers are not as valuable in diagnostics as early markers of the acute-phase reaction, but they inform physicians about inflammation that has been present recently or an infection that has been progressing for a long time.
Negative acute-phase reactants[edit | edit source]
The main examples are albumin, prealbumin, and transferrin. Such markers are diagnostically less important than positive APP for monitoring and evaluating the course of the inflammatory response. However, they are often used as a criterion for protein synthesis in the liver and as indicators of malnutrition.
Immunoglobulins[edit | edit source]
Antibodies (immunoglobulins) are specific blood plasma globulins with β-γ electrophoretic mobility. They are synthesized by plasma cells as a humoral component of the immune response to a particular antigen. An immunoglobulin molecule has the ability to specifically bind the appropriate antigen against which it has been generated for. After binding, an immune complex is formed. In addition, immunoglobulins perform other functions including, for example, complement binding, binding to neutrophilic leukocytes and macrophages, activation of phagocytosis. Immunoglobulins are divided into 5 classes IgG, IgM, IgA, IgD, and IgE. In addition, the IgG class is further divided into 4 subclasses - IgG-1, IgG-2, IgG-3, IgG-4. Also, there are 2 IgA subclasses: IgA-1 and IgA-2. The basic structure of an immunoglobulin molecule consists of two identical heavy chains (H-chains), denoted by the individual classes γ, μ, α, δ, and ε, and two light chains (L-chains): κ and λ, which are common to each class. Each immunoglobulin molecule contains either κ or λ chains, not both. During the first infection with bacteria or protozoa, IgM antibody production occurs within 2 to 3 days. This is eventually replaced by IgG production with the same specificity within 5 to 7 days. Subsequent infections will cause a rapid increase in IgG levels and a small increase in IgM levels. Significant changes in the concentration of immunoglobulins manifest in electrophoretic examination such as in:
- hypogammaglobulinemia (reduction of the peak in the γ zone)
- hypergammaglobulinemia
- polyclonal (broad base β-γ globulin peak increase)
- monoclonal (narrow peak in the region of β-γ globulins)
Hypogammaglobulinemia[edit | edit source]
Hypogammaglobulinemia (decreased levels of immunoglobulins) results from decreased immunoglobulin synthesis or increased immunoglobulin losses (via urine or stool). It can be an isolated defect of a particular class of immunoglobulins, but it can also be a defect of all classes. These humoral immunity defects can be primary or secondary and are the cause of severe immunodeficiency conditions manifested by recurrent, severe infections.
Methods of assessing the protein concentrations[edit | edit source]
A basic measurement of proteins in plasma or serum is the assessment of total serum/plasma protein. However, this examination's merits are rather limited. More information can be retrieved if the proteins are first separated (usually via electrophoresis) and then quantified.
Serum protein electrophoresis[edit | edit source]
Principle[edit | edit source]
Serum protein electrophoresis (SPEP) is a separation method based on the movement of charged particles within an electric field. The compounds of interest need to be charged (i.e. they must either be ions, or ampholytes). Most proteins have ampholytic nature; therefore, their net charge can be positive or negative with the variance of pH of the buffer during electrophoresis. Once a mixture of various charged molecules is exposed to a stationary electric field, individual ions will start moving towards either electrode. The velocity of movement of ions depends on the following factors:
- the charge of the molecule (positive ions move towards the negative electrode, while negative ions move towards the positive electrode)
- the magnitude of the charge (the higher charge, the more the molecule is attracted to the oppositely charged electrode. If the net charge of a molecule is equal to zero, the molecule will not move at all)
- size or relative molecular mass of the compound (molecules with higher molar mass will move slower than those with lower molar mass)
- voltage
Usually, a mixture of protein is separated during electrophoresis at pH 8.6 (using alkaline buffer). Because the isoelectric point of most serum proteins is near 5 to 6, at pH 8.6, all proteins are negatively charged, therefore, they will move towards the anode (positive electrode).
Blood serum electrophoresis usually separates proteins into 6 to 7 fractions: prealbumin (seen rarely), albumin, α1, α2, β1, β2, (sometimes poorly resolved, may be seen only as β fraction), and γ fractions. With the exception of albumin and prealbumin fractions, which contain only single protein each, these fractions consist of multiple proteins with similar electrophoretic mobilities.
Plasma protein concentration by electrophoretic fraction[edit | edit source]
| Fraction | Relative protein concentration (%) | Absolute protein concentration (g/L) |
|---|---|---|
| Albumin | 55 to 69 | 35 to 44 |
| α1 | 1.5 to 4 | 1 to 3 |
| α2 | 8 to 13 | 5 to 8 |
| β | 7 to 15 | 4 to 10 |
| γ | 9 to 18 | 5 to 12 |
Clinical use[edit | edit source]
Serum protein electrophoresis (SPEP) is used especially after a pathologic finding during total protein examination, or if more detailed information about serum proteins is needed for any other reason. Important scenarios that can be observed by electrophoresis of serum proteins:
- dysproteinemia - change in the concentration and qualitative composition of individual proteins in serum,
- paraproteinemia - characterized by the presence of monoclonal immunoglobulins.
[edit | edit source]
| Electrophoresis results | Comment | Alb | α1 | α2 | β | γ | Examples of common pathologies |
|---|---|---|---|---|---|---|---|
| Acute inflammation |
|
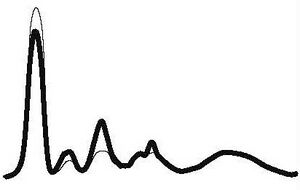
|
| ||||
| ↓ or N | ↑ | ↑ | N | ||||
| Chronic inflammation |
|
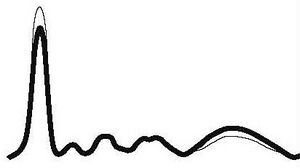
|
| ||||
| ↓ or N | N | N | N | ↑ | |||
| Chronic active inflammation |
|
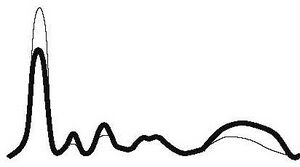
|
| ||||
| ↓ | ↑ | ↑ | N | ↑ | |||
| Hepatic type |
|
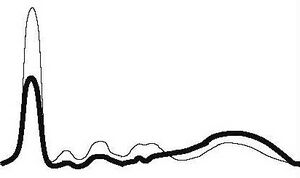
|
| ||||
| ↓ | ↓ | ↓ | ↓ | ↑ | |||
| Nephrotic type |
|
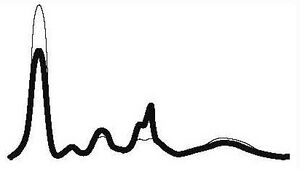
|
| ||||
| ↓ | N | ↑ | ↑ | ↓ or N | |||
| Hypogammaglobulinemia |
|

|
| ||||
| N | N | N | N | ↓ | |||
| Monoclonal gammopathy |
|

|
| ||||
| ↓ | ↓ | ↓ | ↑ | ↑ | |||
Urine proteins[edit | edit source]
Physiologically, urine contains a very small amount of protein that is not detectable by basic precipitation or colorimetric techniques – we speak of so-called physiological proteinuria. If there is a larger amount of protein in the urine, it is proteinuria in the narrower sense of the word.
Physiological proteinuria[edit | edit source]
A healthy adult normally excretes about 50-80 mg of protein, maximum 96 mg/m2 (i.e. about 150 mg) in 24 hours (physiological proteinuria) in the urine during normal physical activity. About 40% of these proteins are plasma proteins and the remaining 60% are renal proteins (Tamm-Horsfall uromucoid) and urinary tract proteins (secretory IgA, unspecified glycoproteins, and glycopeptides).
The amount and composition of proteins in urine depend on their filtration through the glomerular membrane, resorption in tubular cells and renal haemodynamics.
Normally, the glomerular membrane prevents the excretion of substances with a molecular weight of Mr > 60 000.
In glomerular ultrafiltration, two essential factors apply:
- The microporous structure of the glomerular basement membrane prevents the penetration of plasma proteins with Mr greater than 100 000-150 000 (e.g. IgG and IgA). We refer to size selectivity.
- Electrostatic barrier' is determined by the surface of all structural components of the glomerular wall. Hydrated polyanionic macromolecules of glomerular wall structures exhibit a predominant negative charge. Most plasma proteins also have a negative charge that causes them to be repelled. This mechanism prevents the permeation of proteins even with molecular weights around 60 000-70 000 (e.g. albumin and transferrin). Neutral or even positively charged molecules pass through the glomerular filter much more easily. This is charge selectivity.
Classification of proteinurias[edit | edit source]
The classification of proteinuria is of considerable diagnostic value. Proteinuria can be divided into three basic groups, which are further classified:
- Prerenal.
- Renal:
- glomerular proteinuria,
- selective,
- non-selective,
- tubularproteinuria,
- mixed-proteinuria.
- glomerular proteinuria,
- Postrenal.
Prerenal proteinuria[edit | edit source]
Prerenal proteinuria (overflow proteinuria) occurs at high plasma concentrations of low-molecular-weight proteins, which even under physiological circumstances can pass into the ultrafiltrate. Glomerular permeability of proteins may not be impaired. Tubular resorption may also be normal, but due to the increased load, some protein leaks into the urine because the resorption capacity of the proximal tubule is exceeded.
Prerenal proteinuria occurs e.g. in excessive intravascular haemolysis, when haemoglobin (haemoglobinuria) is present in the urine, or in crush syndrome and rhabdomyolysis, when myoglobin (myoglobinuria) is present in the urine. The presence of immunoglobulin light chains in urine (so-called Bence-Jones protein) is indicative of myeloma. Prerenal proteinuria often accompanies the onset of some acute inflammatory and necrotizing diseases. They are conditioned by the secretion of tissue degradation products and, to some extent, some low-molecular-weight acute phase proteins (e.g. orosomucoid).
Renal proteinuria[edit | edit source]
Renal proteinuria is divided according to the affected part of the nephron into glomerular, tubular, and mixed.
- Glomerular proteinuria
- is a manifestation of increased permeability of the glomerular wall to proteins. In selective glomerular proteinuria, when the negative charge of the glomerular membrane is lost, proteins of intermediate molecular mass with Mr between 70 000-100 000 (especially albumin and transferrin) are excreted into the urine at an increased rate, while proteins of high molecular mass are retained. In the urine, albumin predominates and has the highest negative charge. With more severe damage, the glomerular membrane loses the ability to discriminate proteins by size during filtration, and proteins with Mr above 100 000 such as IgG penetrate the urine in addition to proteins of intermediate size. We are talking about nonselective glomerular proteinuria. In glomerular proteinuria, usually the daily protein loss exceeds 2 g.
- Tubular proteinuria
- is caused by reduced tubular resorption of normally filtered proteins. It is characterized by increased secretion of low-molecular-weight proteins (microproteins) that are resorbed in the tubules under physiological conditions (β2-microglobulin, α1-microglobulin, free immunoglobulin light chains). A very common cause is the tubular damage by some nephrotoxic drugs (cytostatics, some antibiotics, many analgesics, and anti-inflammatory agents) or heavy metals (Hg, Pb, Cd). Tubular proteinuria may accompany some prerenal proteinuria (paraproteinuria, myoglobinuria) because there is a competitive relative lack of tubular resorption of other freely filterable proteins. Protein losses in tubular proteinuria are not large, usually 0.3-1.5 g/24 hours.
Mixed proteinuria is a combination of non-selective glomerular proteinuria and tubular proteinuria. It is a manifestation of the death of most nephrons.
Postrenal proteinuria[edit | edit source]
Postrenal proteinuria occurs in hemorrhage, tumors, and urinary tract infections when there is a direct passage of plasma or protein exudation into the urine. It is reliably identified by the detection of plasma macromolecular proteins of high molecular weight (α2-macroglobulin, IgM), which do not penetrate the wall of the glomerular capillary even in non-selective proteinuria.
| Type of proteinuria | Characteristic proteins in the urine | Notes |
|---|---|---|
| RENAL | ||
| Selective glomerular |
(Mr 70 000–100 000) |
|
| Non-selective glomerular |
(Mr > 70 000) |
|
| Tubular |
(Mr 10 000–70 000) |
|
| Mixed (tj. glomerulo-tubular) |
|
|
| PRERENAL |
|
|
| POSTRENAL |
|
|
Functional proteinuria[edit | edit source]
Functional (also benign) proteinuria is a special form of glomerular proteinuria. It is explained by changes in blood flow through the glomerulus. It is encountered after great physical activity, colds, in fevers, or congestive heart failure[13]. Protein losses do not exceed 1 g/day. In some adolescents, proteinuria occurs only in the upright position, so-called postural (orthostatic) proteinuria, which is often explained by vasoconstriction in lumbar hyperlordosis and is the most common cause of proteinuria in children[14]. In this case, daily protein losses can exceed even 1 g. Most of these proteinuria spontaneously adjust after the provoking cause has resolved. Also, in pregnancy, urinary protein waste increases to 200-300 mg/day.
Albuminuria[edit | edit source]
Urinary excretion of albumin does not physiologically exceed 30 mg/24 hours (i.e. 20 μg/min or about 15-20 mg/l)[15]. However, conventional tests for proteinuria (using diagnostic strips, sulphosalicylic acid test) can only detect protein when the albumin concentration exceeds about 150 mg/l, i.e. when it is practically 10 times elevated[16]. Losses of small amounts of albumin (30-300 mg/24 h) are demonstrable by immunochemical methods.
In older literature, the term microalbuminuria was used for small albumin losses that are demonstrable by immunochemistry but not by conventional proteinuria tests.
Screening for albuminuria is particularly valuable in patients with diabetes mellitus type II, but also in other disorders of glucose metabolism and in hypertensives. The finding of small amounts of albumin in the urine is an early sign of complications of these diseases, especially diabetic or hypertensive nephropathy and vasculopathy, and is often a reason to intensify treatment.
An increase in albuminuria is a very sensitive indicator of damage to the glomerular apparatus. This is due to the fact that albumin in small amounts passes through the glomerular membrane even physiologically. Under normal circumstances, however, it is almost completely resorbed in the proximal tubules. However, the tubular resorption capacity of albumin is virtually exhausted already during physiological albumin filtration; any increase in the concentration of this protein in the glomerular filtrate therefore leads to a rapid increase in the concentration of albumin in the final urine[16].
| Albuminuria | |||
|---|---|---|---|
| mg/24 hour | μg/min | mg/mmol of creatinine | |
| norm | < 30 | < 20 | < 3,5 |
| elevated albuminuria | 30–300 | 20–200 | 3,5–35 |
| detectable proteinuria | > 300 | > 200 | > 35 |
To monitor disease progression and manage treatment, albuminuria needs to be quantified more precisely. Albumin is determined in urine collected overnight and losses are converted to μg of albumin per minute. Values less than 100 μg/min usually correspond to reversible damage, which can be influenced by careful compensation for diabetes and arterial hypertension[15].
Another option is to determine albumin in the first morning urine sample and calculate the albumin/creatinine ratio. Physiologically, this index is around 2.8-22.8 g of albumin per mole of creatinine[16].
For albuminuria testing to be meaningful, it is necessary to exclude uroinfection.
Qualitative determination of protein in urine[edit | edit source]
Diagnostic strips are used to detect pathological proteinuria. In some laboratories, the strip test is combined with a sulfosalicylic acid test.
Diagnostic strips[edit | edit source]
The principle of protein determination in urine using diagnostic strips is based on the so-called protein acid-base indicator error, e.g. tetrabromophenol blue, tetrabromophenolphthalein ethyl ester, or 3´,3´´,5´´,5´´-tetrachlorophenol-3,4,5,6-tetrabromophenolphthalein. Like any acid-base indicator, these substances change colour at a certain pH (they behave like weak acids, with the protonated form having a different colour than the dissociated form): at pH lower than 3.5 they are yellow, at higher pH they are green to blue. In addition to the indicator, the reaction zone of the test strip contains a buffer which maintains the pH between 3,0 and 3,5, so the indicator is yellow. If there are proteins in the sample, they bind to the indicator with their amino groups. However, this changes its properties – the transition region shifts towards the more acidic pH. This means that at the constant pH between 3.0 and 3.5, the indicator with the protein bound will be green, as if it were in a more alkaline environment (hence the protein indicator error). The intensity of the colour depends on the concentration of the protein, ranging from green to blue, and is assessed visually or instrumentally.
For strongly alkaline urines (pH above 8) or if the urine is very concentrated, the test may give false positive results (buffer depletion in the reaction zone). In these cases, we acidify the urine with a few drops of dilute acetic acid to pH 5-6 and repeat the test. False positives can also be caused by high concentrations of certain substances with amino groups (contamination of the collection vessel by certain disinfectants), which bind to the indicators in a similar way to proteins.
The disadvantage of the test strips is their different sensitivity to individual proteins. The strips react very well with albumin and indicate its presence in urine from 0.1 to 0.5 g/l. They show significantly lower sensitivity to globulins, glycoproteins, and Bence-Jones protein. These diagnostic strips cannot demonstrate the increase in albuminuria to values up to about 200 mg/l or the daily albumin loss of 30 to 300 mg/24 hours that accompanies the earlier stages of some nephropathies. Immunochemical methods such as special diagnostic strips based on immunochromatographic principles or immunoturbidimetry can be used to screen for an increase in albuminuria.
Sulfosalicylic acid test[edit | edit source]
The principle of the test is denaturation of the protein by sulfosalicylic acid, which results in opalescence to opacity.
The reaction is sensitive, showing 0.1-0.2 g/l of total proteinuria. Differences in the detection of individual proteins are not as pronounced as with diagnostic strips. Sulfosalicylic acid also precipitates globulins.
False-positive results are given by this test when certain X-ray contrast agents, penicillin, sulphonamides, salicylic acid, and antidiabetic drugs are excluded.
A semi-quantitative scale is used for evaluation:
| Finding | Evaluation | Approximate concentration of protein in g/l |
|---|---|---|
| Opalescence | trace amounts | 0,05–0,1 |
| Light haze (transparent, underlying text is readable) | + | 0,1–0,2 |
| Milky haze (opaque, without flakes) | ++ | 0,5–1,0 |
| Milky haze with flake formation | +++ | 2,0–5,0 |
| Flaky clot | ++++ | ≥ 5,0 |
Quantitative determination of protein in urine[edit | edit source]
Quantitative determination of protein in urine is methodologically quite difficult. In clinical-biochemical practice, methods are used for the determination of proteinuria, which can be divided into three groups according to the principle:
- methods based on denaturation followed by turbidimetric determination of the haze (e.g. methods with trichloroacetic acid or sulfosalicylic acid);
- colorimetric methods with or without prior denaturation of proteins (e.g. biuret reaction);
- methods based on binding of dyes to proteins (e.g., the method with Coomassie brilliant blue G 250 according to Bradford, with pyrogallol red, etc.).
Today, techniques that can be automated - turbidimetry and colour reaction with pyrogallol red - are preferred.
Determination of proteinuria using pyrogallol red[edit | edit source]
Pyrogallol red is used for protein quantification, e.g. for the determination of proteinuria. Pyrogallol red forms a pink complex with molybdenum with an absorption maximum at 470 nm. When the protein binds to this complex in an acidic environment, the colour deepens and the absorption maximum shifts to the 600 nm region. The absorbance of the resulting complex of reagent with protein is linearly dependent on the concentration of protein in the sample in the concentration range 0.06-2.0 g/l.
Assessment of the magnitude of proteinuria[edit | edit source]
| < 0.150 g/24 hod | physiological proteinuria |
| < 1 g/24 hod | low proteinuria (mostly tubular) |
| 1.0–3.5 g/24 hod | medium proteinuria |
| > 3.5 g/24 hod | high proteinuria |
| > 10 g/24 hod | proteinuria usually associated with severe nephrotic syndrome |
Typification of proteinuria[edit | edit source]
To determine the type of proteinuria, it is necessary to know the spectrum of proteins excreted in the urine. Electrophoretic methods are used for this purpose. Electrophoretic partitioning of urinary proteins according to their molecular weight allows semiquantitative evaluation of individual diagnostically significant proteins and classification of proteinuria. Electrophoresis in agarose or polyacrylamide gel has gradually become the method of choice for urinary protein analysis.
In order to separate the proteins by size (rather than charge), a polyacrylamide gel can be used, the density of which increases from the cathode to the anode (i.e., the mesh or pore size in the gel gradually decreases). Small molecules in such a gel travel further than large molecules.
Another, more commonly used option, is to treat the sample with the detergent sodium lauryl sulfate (sodium dodecyl sulfate – SDS), which "surrounds" the protein and replaces its own charge with its negative charge. The resulting complexes have approximately the same charge (more precisely: they have the same surface charge density). If electrophoresis is then carried out in a relatively dense gel, it is separated according to relative molecular weight: smaller molecules travel through the gel faster than large ones (molecular sieve technique). It moves the fastest β2-microglobulin, albumin lies about midway along the partition pathway; between the start and albumin are proteins with Mr greater than 70 000.
Urinary protein electrophoresis evaluation[edit | edit source]
In glomerular proteinuria we find proteins in the electrophoreogram between start and albumin (i.e. Mr > 70 000).
Proteins observed in glomerular proteinuria Mr Albumin 68 000 selective non-selective Transferrin 77 000 selective non-selective IgG 150 000 non-selective IgA 160 000 non-selective Haptoglobins 85 000–1 000 000 non-selective
Tubular proteinuria are characterized by the presence of protein between albumin and the anodic end of the electrophoreogram (i.e. Mr < 70 000).
Proteins observed in tubular proteinuria Mr β2-microglobulin 11 800 Lysozyme 15 000 Retinol binding protein (RBP) 21 000 Ig free light chains 25 000 α1-microglobulin 33 000 Dimeric Ig free light chains 50 000 Albumin 68 000
Proteins observed in tubular proteinuria
Presence of α2-macroglobulin (Mr = 800 000) with other findings is similar to mixed proteinuria suggest postrenal proteinuria.
Links[edit | edit source]
Related articles[edit | edit source]
External links[edit | edit source]
- ↑ BURTIS, Carl A a Edward R ASHWOOD. Tietz textbook of clinical chemistry. 2. vydání. Philadelphia : Saunders, 1994. 2326 s. ISBN 0-7216-4472-4.
- ↑ ŠVÍGLEROVÁ, Jitka. Albumin [online]. Poslední revize 2009-02-18, [cit. 2010-10]. <https://web.archive.org/web/20160416224413/http://wiki.lfp-studium.cz/index.php/Albumin>.
- ↑ RACEK, Jaroslav, et al. Klinická biochemie. 2. vydání. Praha : Galén, 2006. 329 s. s. 71. ISBN 80-7262-324-9.
- ↑ a b ZIMA, Tomáš, et al. Laboratorní diagnostika. 2. vydání. Praha : Galén a Karolinum, 2007. 906 s. ISBN 978-80-246-1423-6.
- ↑ KESSLER, Siegfried. Laboratorní dagnostika. 1. vydání. Praha : Scientia medica, 1993. 252 s. Memorix; s. 52. ISBN 80-85526-12-3.
- ↑ KESSLER, Siegfried. Laboratorní dagnostika. 1. vydání. Praha : Scientia medica, 1993. 252 s. Memorix; s. 52. ISBN 80-85526-12-3.
- ↑ ZIMA, Tomáš, et al. Normální hodnoty [online]. Velký lékařský slovník online, [cit. 2020-02-13]. <http://lekarske.slovniky.cz/normalni-hodnoty>.
- ↑ GREGOR, Pavel a Petr WIDIMSKÝ, et al. Kardiologie. 2. vydání. Praha : Galén, 1999. 595 s. s. 168. ISBN 80-7262-021-5.
- ↑ KLENER, Pavel, et al. Vnitřní lékařství. 3. vydání. Praha : Galén a Karolinum, 2006. 1158 s. ISBN 80-7262-430-X.
- ↑ LIU, H. H., J. B. GUO a Y. GENG. Procalcitonin: present and future. Irish Journal of Medical Science (1971 -). 2015, roč. 3, vol. 184, s. 597-605, ISSN 0021-1265. DOI: 10.1007/s11845-015-1327-0
- ↑ a b c ÚKBLD 1. LF a VFN Praha. Prokalcitonin : vývoj názorů na interpretaci [online]. ©2009. [cit. 2011-06-30]. <http://www.cskb.cz/res/file/akce/sjezdy/2009-Pha/ppt/B1/Kazda.pdf>.
- ↑ STRICKLAND, RD, ML FREEMAN a FT GURULE. Copper binding by proteins in alkaline solution. Analytical chemistry [online]. 1961, vol. 33, no. 4, s. 545-552, dostupné také z <https://pubs.acs.org/action/cookieAbsent>. ISSN 0003-2700. DOI: 10.1021/ac60172a019.
- ↑ BURTIS, Carl A – ASHWOOD, Edward R – BRUNS, David E. Tietz textbook of clinical chemistry and molecular diagnostics. 4. edition. St. Louis, Mo : Elsevier Saunders, 2006. 2412 pp. pp. 576. ISBN 978-0-7216-0189-2.
- ↑ MUNTAU, Ania Carolina. Pediatrics. 4. edition. Grada, 2009. pp. 420. ISBN 978- 80-247-2525-3.
- ↑ a b ZIMA, Tomáš, et al. Laboratorní diagnostika. 2. edition. Praha : Galén a Karolinum, 2007. 906 pp. pp. 106-7, 121-2. ISBN 978-80-246-1423-6.
- ↑ a b c RACEK, Jaroslav, et al. Klinická biochemie. 2. edition. Praha : Galén, 2006. 329 pp. pp. 170. ISBN 80-7262-324-9.
- ↑ KRAML, Jiří, et al. Návody k praktickým cvičením z lékařské chemie a biochemie. 1. edition. Praha : Karolinum, 1991. 312 pp. pp. 243. ISBN 80-7066-453-3.