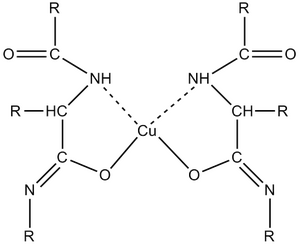
Biuret reaction
In an alkaline environment in the presence of copper salts give the proteins a purple colour, suitable for photometric determination[1] [2]. In simple terms, complex compounds of Cu2+ with peptide bond ions are formed[3]. The formed complex strongly absorbs light in the 540-560 nm region. The intensity of the colour of the complex is measured photometrically and the absorbation is directly proportional to the concentration of protein. The biuret reaction (so-called biuret test) is generally provided by substances containing at least two peptide bonds (-CO-NH-) or two -CO-NH2 groups in molecule. Thus, the reaction is not specific to proteins only. The simplest compound reacting with copper salts in an alkaline environment is biuret containing two peptide bonds. Amino acids and dipeptides do not react.
The biuret reagent includes copper sulfate, which provides Cu2+ for the formation of complexes with peptide bonds, and an alkalizing component (hydroxide), which converts the peptide bond to an enol form, allowing oxygen atoms to participate in the complex. Other components of the reagent are potassium sodium tartrate, whichas a complexing agent prevents precipitation Cu2+ to Cu(OH)2, and potassium iodide, which protects Cu2+ from autoreduction. The sensitivity of the biuret method is around 1-10 g protein/l.
Sources[edit | edit source]
Related articles[edit | edit source]
References[edit | edit source]
- ↑ DOUMAS, B T. Standards for total serum protein assays--a collaborative study. Clin Chem [online]. 1975, vol. 21, no. 8, p. 1159-66, Available from <https://www.ncbi.nlm.nih.gov/pubmed/1169135>. ISSN 0009-9147.
- ↑ DOUMAS, B T – BAYSE, D D – CARTER, R J. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin Chem [online]. 1981, vol. 27, no. 10, p. 1642-50, Available from <https://www.ncbi.nlm.nih.gov/pubmed/6169466>. ISSN 0009-9147.
- ↑ STRICKLAND, RD – FREEMAN, ML – GURULE, FT. Copper binding by proteins in alkaline solution. Analytical chemistry [online]. 1961, vol. 33, no. 4, p. 545-552, Available from <https://pubs.acs.org/action/cookieAbsent>. ISSN 0003-2700. DOI: 10.1021/ac60172a019.