Viral infections of the nervous system
CNS viral infections usually occur within generalized viruses. Multiple nerve structures are often damaged at the same time.
- gateway of viral infection: respiratory tract, gastrointestinal tract, urogenital tract, skin
- the infection spreads to the CNS along the peripheral nerves / hematogenously
- involvement: meninges → meningitis, brain and spinal cord tissues → encephalitis or myelitis, anterior horns → poliomyelitis, sensitive spinal ganglia of spinal cord → radiculitis
- acute to chronic infections, the virus can survive in nervous structures for years asymptomatically, a number of virosis seasonal occurrence
- immunosuppression and cytostatics create the conditions for virus replication
- active immunization (today eg rare polioviruses)
- diagnosis: detection of virus from cerebrospinal fluid, faeces, nasopharynx, increase in antibody titer in fluid or blood
- treatment of most viruses only symptomatic, exception: herpes viruses - aciclovir
- antiviral sera are available for some diseases, active immunizations are given for others [1]
Viral meningitis
Viral meningitis is the most common viral infection of the CNS along with inflammation of the soft envelope of the brain (leptomeningitis). Aseptic meningitis includes, in addition to viral meningitis, other forms of meningitis where culture does not reveal the causative agent. The incidence of viral meningitis is difficult to monitor, but it is reported to affect about 1 in 3000 patients with viremia .[2]
Etiology
The most common causative agents are
- enteroviruses - currently more than 85% of cases),
- mumps virus,
- HSV 2,
- EBV,
- rarely lymphocytic choriomeningitis, AIDS.
Pathogenesis
The viral pathogen can enter the CNS in 2 ways: hematogenously (most common) and neurogenically (typical of herpesviruses)[2];
- In the spring period, young patients are affected by mumps virus; parotitis can be complicated by meningitis, there is also a possibility of residual hearing loss;
- mononucleosis is caused by EBV, affects adolescents, may be complicated by serous meningitis, monocytosis in the blood count, increased fatigue lasting several months!;
- enteroviral infections (caused by ECHO viruses) occur seasonally in adolescents in the summer and autumn, spread by the fecal-oral route, mainly affect the children under one year of age, more common in lower social groups;
- Coxsackie viruses cause C-viruses with severe muscle pain;
- HSV 2 is responsible for 5% of viral meningitis, ¼ of these patients have primary genital infection;
- lymphocytic choriomeningitis is spread by airborne transmission from rodent feces;
- HIV infection is thought of in high-risk groups; HIV antibodies appear 1-3 months after the onset of illness.
Clinical signs
- 1) Prodromal phase (flu) - fever, muscle and throat pain, diarrhoea, exanthema, fatigue and malaise; lasts 3-7 days
- Latency phase - 2-5 days with virtually no difficulties
- 2) Neuroinfection phase - photophobia, headache, nausea to vomiting, dizziness, meningeal symptoms, somnolence;
- recovery usually within 2 weeks;
- some cases may proceed asymptomatically or only through the first phase.
Pathological-anatomical pattern
- Inflammation causes edema, engorgement of the meninges and their infiltration by lymphocytes;
- it also affects the surface of the brain (spinal cord).
Differential diagnosis
- Subacute onset distinguishes a group of clinically severe meningitis;
- Tuberculous and mycotic meningitis, leptospirosis, sarcoidosis, meningocarcinomatosis, partially treated bacterial meningitis;
- the mild, easy course and spontaneous recovery of acute meningitis differs from the severe course and prognosis of these subacute and chronic meningitis.
Auxiliary examinations
- CSF from Lumbar puncture fluid: proteinocytological association, predominance of lymphocytes over monocytes, mild proteinorachia;
- Virus culture from: CSF (early collection), nasal swab, throat swab, stool;
- rise in serological titres in the acute phase of the virus compared to convalescence;
- sedimentation usually normal, leukocytosis at most mild;
- the course of the disease mild;
- virus identification fails in about 20-40%.[3]
Treatment
- Symptomatic and supportive with rest and vitamin supplementation;
- acyclovir i.v. - when HSV type 1 or 2 is detected;
- prognosis is always good (self-limiting disease with complete recovery in 7-10 days).
Viral encephalitis
They occur most in the tropics. Viruses affect the brain in 4 ways:
- directly (acute encephalitis or meningoencephalitis)
- after various latencies ("slow" viral encephalitis)
- indirectly via the immune system (allergic, post-infectious or post-vaccination encephalomyelitis)
- encephalopathy (Reye's syndrome) also develops within the viral infection
Acute viral encephalitis
The result of acute viral encephalitis are neuronal and glia defects occurring with inflammation and edema.
Etiology
- Mumps, HSV, VZV, EBV.
- Substantial link to the vector: tick – tick encephalitis, Russian spring encephalitis; mosquito - western equine disease (USA), western Nile encephalitis (Africa).
- Post-infectious encephalitis – follow childhood infections (measles, chickenpox, rubella), it is not a direct effect of the virus.
Clinical manifestations
- General manifestations ("influenza"): muscle pain, fever, headache, meningeal reactions, cerebrospinal fluid cell proliferation.
- Symptoms of brain impairment - focal/diffuse, according to location.
- With hemisphere involvement → epilepsy, involuntary movements, paresis, confusion, speech disorders.
- Rhombencephalits = defects in cerebellar and brainstem structures.
- Mesencephalic defect→ oculomotoric and autonomic disorders.
- Cerebellar defect→ ataxia, dysarthria.
- Brainstem defect → nystagmus, quadruparesis, cranial nerve palsy.
- Spinal cord defect→ mixed motor, sensitive and autonomic dysfunction.
Prognosis
- Usually lasts for several weeks;
- the prognosis depends on the type of virus;
- mortality HSV infection 20-30%, in mumps only 2%;[4]
- also the neurological consequences vary in severity.
Tick-borne encephalitis
Tick-borne encephalitis (TBE) is a disease caused by the tick-borne encephalitis virus (TBEV), which is an arbovirus. The disease can lead to meningitis, meningoencephalitis, and severe encephalomyelitis. The course is variable- from abortive forms (with few symptoms) to a typically two-phase course with central nervous system involvement. The clinical picture manifests as a febrile illness with headaches and neurological symptoms. There is no specific antiviral therapy: the treatment is only symptomatic. TBE mortality is low, but permanent neurological sequelae are relatively common. It is possible to vaccinate against TBE.
TBEV is one of the most common causes of aseptic neuroinfections in the Czech Republic. The clinical picture of meningitis prevails in children with up to 2/3 of children showing cognitive deficits after having KME and have memory problems.
Epidemiology
Tick-borne encephalitis is an endemic local seasonal neuroinfection.
- Originator: arbovirus (arthropod-borne virus, enveloped RNA virus) of the family Flaviviridae.
- Infection reservoir: small rodents and larger forest animals, sheep, goats.
- Transmission (vector): by sucking blood of infected nymphs or adult ticks (Ixodes ricinus)
- the virus is in the saliva of ticks, so a short suction time is enough to transmit the virus.
- the virus survives in the salivary glands of the tick and in the tick there is also a transovarian transmission of the virus
- rarely, transmission is via the alimentary route- by consuming unpasteurized milk from infected goats and sheep.
- Incubation period: 3-28 days.
The reported incidence of tick-borne encephalitis in the Czech Republic in the years 2000–2009 is 500–1000 cases per year, i.e., 5-10 patients per 100,000 population per year. It has been known in the Czech Republic only since 1945 and its occurrence is most frequent in the Vltava, Berounka, and Sázava river basins, in Central and Southern Bohemia, most often from April to October.
Clinical symptoms and course
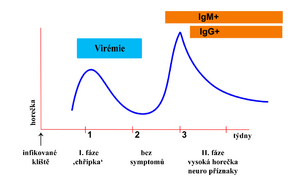
The incubation period of the disease is 7–14 days, with an extreme variation of the duration of symptoms (3–30 days). Most infections are inapparent. After 1-3 weeks of incubation, a two-phase course is usually typical:
- Phase 1 ("flulike") – Viremia with headache and muscle pain, fever, fatigue. The condition improves in a few days. An apparent recovery in the form of an afebrile period (2-7 days) follows. The asymptomatic period that lasts 1–20 days.
- Phase 2 – meningeal symptoms: headache, photophobia, encephalomyelitic symptoms: alterations of consciousness (sleepiness to coma), cranial nerve disorders, bulbar syndrome, weak limb paresis, high fever, sleep disturbance, vomiting, and tremors.
Based on the predominant disability, we can divide encephalitis into several forms:
- inapparent (specific antibody production only)
- abortifacient (nonspecific symptoms similar to influenza illness)
- meningeal (viral meningitis)
- encephalic (gray and white brain disease with neurological symptoms)
- encephalomyelitic (involvement of gray, white matter and anterior horns); weak paresis, especially of the brachial plexus as segments C5–7 are most often affected by the process.
- bulbocervical (involvement of the medulla oblongata), bulbocervical forms can also lead to the failure of vital centers and thus to death.
Diagnosis
- positive meningeal symptoms in people living in the endemic area;
- a history of a typical two-phase course;
- tick bite data - indicates only a part of patients with TBE;
- detection of specific antibodies from serum - ELISA with detection of early IgM antibodies, IgG class antibodies are formed very quickly, for which their avidity can be determined
- other serological methods: specific virus neutralization test, rise of specific antibodies
- cerebrospinal fluid examination: aseptic inflammation with a leukocyte count ranging from 100-200 leukocytes/μL, slightly elevated protein levels
- EEG in the acute phase: diffuse pathological recording with a predominance of slow waves.
Treatment
So far it is only symptomatic (analgesics , antiemetics , antipyretics). Resting is especially important. Relieving lumbar puncture can be performed (tens to hundreds of lymphocytes in the CSF, slightly higher protein). We treat paresis by administering vit. B and rehabilitation. Anti-edematous treatment (mannitol) and corticoids also have a positive effect. It is recommended to avoid the sun, prolonged television and higher mental strain.
Prevention
Vaccination with an inactivated virus vaccine (FSME-IMMUN (Baxter) approved since 1976 and Encepur (Novartis) approved since 1991). The basic vaccination schedule consists of 3 doses. Vaccines are well tolerated, the most common adverse reactions being a fever in the range of 38.0-39.0 °C (20% of children; most often in the age group of 1-3 years; most in the period from February to March, i.e., in the period of frequent respiratory infections), injection site pain and, rarely, muscle weakness. The World Health Organization recommends vaccination against tick-borne encephalitis to all people living in the endemic area, including children.
The disease prevention includes regimens including appropriate clothing covering the entire body, the use of repellents, early removal of the tick and disinfection of the injection site.
Drinking of pasteurized milk is also a prevention (it is also spread by the milk of infected animals, including cow's milk).
Prognosis and consequences
More severe forms have convalescent period lasting weeks to months. Residual symptoms persist (in 10% of patients) in the form of peripheral weak paresis, and memory, concentration, and sleep disorders.
Herpetic meningoencephalitis
- HSV typ I – herpes labialis et oralis – encefalitis.
- HSV typ II – herpes genitalis –serous meningitis in newborns.
Etiology
- Herpes DNA viruses include: HSV types I and II, VZV, EBV, CMV;
- capable of long-term latent survival in nervous tissue with the possibility of exacerbation infection;
- transmission throughout the year in all age groups - by contact, by air;
- HSV type I
causes severe hemorrhagic-necrotic encephalitis selectively affecting the frontotemporal area, 30% mortality
- HSV typ II
- causes serous meningitis - like other viral meningitis.
Pathological-anatomical picture
- Necrotizing encephalitis (necrosis most often in the cortex of the frontal lobes);
- brain severely swollen at autopsy, sanguineous meninges;
- intranuclear eosinophilic inclusion bodies;
- biopsy shows antigen herpes simplex by immunofluorescence and positive culture within 48 hours.
Clinical picture
- Non-specific headaches and fever - progress in a few days to seizures with impaired consciousness, focal symptoms correspond selectively to the lower parts of the frontal and temporal lobes;
- olfactory hallucinations, partial seizures with complex symptomatology, behavioral changes;
- involvement of the dominant hemisphere - aphasia - one of the main symptoms;
- cerebral edema can cause death (tentorial herniation);
- survival associated with memory defect.
Diagnosis
- In the acute phase brain biopsy;
- after initiating treatment a non-specific positive CT or MRI finding is sufficient;
- at first, normal CT will show frontotemporal hypodensity (necrosis);
- cerebrospinal fluid - multiplication of lymphocytes, higher protein;
- EEG - generalised slowdown with periodic discharges temporally
Diferential diagnostics and therapy
- Dif. dg.: other encephalitis, brain abcess, tumor; jiné encephalitidy, absces mozku, tumor;
- Unlike other viral CNS infections, we have a causal drug - aciclovir.
- aciclovir should always be used immediately if a herpes etiology of encephalitis is suspected, till proven othervise
Links
Similar articles
References
- BENEŠ, Jiří, et al. Infekční lékařství. 1. vydání. Galén, 2009. 651 s. s. 179, 527-528. ISBN 978-80-7262-644-1.
- ↑ SEIDL, Zdeněk a Jiří OBENBERGER. Neurologie pro studium i praxi. 1. vydání. Praha : Grada Publishing, 2004. ISBN 80-247-0623-7.
- ↑ Skočit nahoru k: a b POVÝŠIL, Ctibor a Ivo ŠTEINER, et al. Speciální patologie. 2. vydání. Praha : Galén-Karolinum, 2007. s. 297-299. ISBN 978-80-7262-494-2.
Source
Herpetic meningoencephalitis is one of the most serious meningoencephalitis in children and adults. Even with the possibility of causal therapy, the diagnosis is often delayed, the disease has a high mortality and the survivors have a high percentage of permanent neurological consequences.
Etiopatogenesis
Etiology
Human herpesvirus is a DNA virus that includes two important strains:
- Human herpesvirus 1 (HHV-1), known as herpes simplex virus 1 (HSV-1), which causes herpes gingivostomatitis that responds to antiviral therapy but even without treatment, is a self-limited disease. In addition to local infections, HSV-1 and HSV-2 are the cause of severe encephalitis. It is more common in adulthood.
- Human herpesvirus 2 (HHV-2), known as herpes simplex virus 2 (HSV-2), causes genital lesions. It is the cause of neonatal encephalitis associated with maternal genital infection.
- HHV transmission: saliva, genital secretions, blood and vertically. The gateway is most often the respiratory tract, damaged skin and genital mucosa. The virus can also enter the macroorganism intravenously or transplacentally.
- In HSV-1, in primary infections are most common: primary gingivostomatitis, pharyngitis, keratoconjunctivitis and encephalitis.
- When the virus is reactivated in herpes labialis in immunosuppressed patients these things may occur: herpetic stomatitis or even esophagitis or even dissemination to generalization of infection.
- In HSV-2, we encounter neonatal encephalitis and genital herpes.
However, HSV-1 can cause diseases more typical of HSV-2 and vice versa.
HSV primarily affects the medial temporal cortex as well as the frontal and parietal lobes.
Pathogenesis of the encefalitis
- It is not sufficiently clarified in humans.
- In animal models, the virus is thought to pass to the CNS through peripheral nerves. Virus-induced apoptosis may then play a role in the molecular pathogenesis of the disease.
- Encephalitis mainly affects the temporal lobes (60%), separate extratemporal involvement is observed in about 15% of patients.
- In children, herpetic encephalitis is often a manifestation of herpetic primary infection (approximately 80% of children do not have a history of labial herpes). In neonates, the infection occurs during the passage through the birth canal, with a higher risk of infection when the mother acquired herpes during pregnancy. Mothers with pre-existing and recurrent genital herpes have a significantly lower risk.
- It is known that herpes viruses, during primary infection, incorporate their DNA into the genome of host lymphoid or nerve cells. In the latent phase of infection, viral replication in the ganglia is minimal, but weakening of immunological surveillance, especially of specific cellular immunity, can lead to reactivation of the latent infection, which is usually much less intense than primary infection.
Clinical picture
Development usually occurs within a few hours to a few days. CNS infliction can be clinically manifested either as a mild disease (aseptic meningitis) or more often as a serious disease state - encephalitis. Likewise, the beginning can be creeping or peracute.
- Prodromal symptoms include fatigue, fever (found in most patients), headache, nausea, vomiting.
- This symptomatology is followed by acute or subacute development of encephalopathy (behavioral disorders, lethargy or irritability, confusion, dysphasia, aphasia, photophobia), hemiparesis, cranial nerve deficits (paresis nn. VII., VI., III.), Visual field defects , paraesthesia, central limb paresis, vegetative disorders.
- Focal neurological findings and focal convulsions usually appear suddenly at the onset of the disease and persist for up to one week. We find focal convulsions in up to 40% of patients. Meningeal signs may be present, but are not common.
- In newborns and infants, we observe highly tuned crying, jaundice, respiratory distress, liver dysfunction.
- In older children, we can observe personality changes, behavioral disorders, memory changes, dysosmia, dysgeusia, hallucinations, bizarre ideas. These symptoms may appear early in the course of the disease and persist for about a week. They can often act as the onset of an acute psychiatric illness.
- In some patients, we observe a peracute course with progression of impaired consciousness leading to death without signs of focal neurological symptoms.
- Rare symptoms include the development of subacute encephalitis, which mimics psychiatric illness or benign recurrent meningitis. Rarely, HSV-1 can cause brainstem encephalitis, HSV-2 myelitis.
- In children who survived HSV encephalitis we may encounter severe antegrade amnesia (quickly forgotten learned) or severe retrograde amnesia (personal knowledge and orientation in time, interval even several years, some has poor memories of faces, many have language difficulties), there are also signs of frontal damage with behavioural disorders.
Diagnostics
When HSV encephalitis is suspected, diagnostic tests should be performed as soon as possible and we should never delay the start of therapy.
In the diagnosis of herpetic encephalitis, HSV cerebrospinal fluid PCR and CNS MRI have the highest yield!
- Basic laboratory tests are not helpful in diagnosing herpes infections, but they are essential because they can rule out other causes of the disease.
- Due to the symptomatology, a CT scan is often necessary in the introduction to rule out a focal lesion of a different etiology. In the diagnosis of HSV encephalitis, CT is evident after 3rd-4th the day of the disease, when it can detect necrotic changes in the temporal area. This option was used earlier, when earlier diagnosis was impossible, but always meant an ex post diagnosis.
- Today, it is replaced by early diagnostics using PCR and MRI.
- The cornerstone of diagnosis and examination are the lumbar puncture and cerebrospinal fluid examination. In the introduction we demonstrate a typical serous pattern with:
- mild elevation of proteinorrhea;
- normal glycorhachia;
- moderate pleiocytosis with a predominance of mononuclear cells.
- We can also find the presence of erythrocytes and xantochromatic appearance. Spectrophotometry eliminates artificial bleeding during the actual puncture.
- It is necessary to send cerebrospinal fluid for PCR detection of DNA for HSV-1 and HSV-2. HSV PCR is highly specific, with positivity remaining 5 days after initiation of acyclovir therapy. On the other hand, for the first 1-2 days and after two weeks of illness, the result may be false negative. The yield of cerebrospinal fluid examination is proportional to the amount of cerebrospinal fluid collected ("the more, the better"), we should usually obtain> 10 ml of cerebrospinal fluid. Despite the undeniable importance of PCR, it is necessary to realize that even this method does not have 100% sensitivity and specificity. In addition, the "quality" of the results varies according to the experience of the laboratories. Therefore, the diagnosis should always be made with regard to clinical symptomatology and further examination. False-negative results are rare, so many authors consider a negative HSV PCR result in the initial cerebrospinal fluid sample as sufficient evidence to discontinue acyclovir treatment.
- CSF antibody serology may support evidence of antibody response in the CNS.
- CT scan and MRI: MRI is more sensitive than CT and is now the imaging method of choice. In MRI we find most changes in the medial area of the temporal lobe and at the base of the frontal lobes, the lesions are often associated with cerebral edema, they are bilateral but rarely symmetrical. Gyrus deletion due to edema in the T1 image and high signal in the T2 image are considered to be early signs of the disease in the MRI image. After application of the contrast agent, enhancement may be found in the gyros. In neonatal HSV-2, we find panencephalitis.
- Another sensitive test for herpetic encephalitis is EEG. Indicates abnormality in 4/5 patients. We observe focal temporal changes and diffuse retardation of activity. Periodic high-voltage spikes - waves originating from the time domain and complexes of slow waves at 2-3 s intervals - are very telling for herpetic encephalitis. Normal EEG recording is rare, but does not rule out the presence of encephalitis.
- At some workplaces it is possible to perform tomographic isotope methodology using Tc - 99m = SPECT.
- Brain biopsy is an outdated diagnostic method.
Differential diagnostics
In addition to bacterial etiology (borrelia, leptospira) and encephalitis caused by other herpes viruses, it is necessary to exclude other possible agents such as:
We must rule out post-infectious and disseminated encephalomyelitis, which follows the viral disease - neurons are not directly infected with the virus, perivenous inflammation and demyelination located in the white matter are in the foreground (most common agents: EBV, CMV, HHV-6).
Cerebellitis (ataxia, tremor, vertigo, dysarthria, vomiting, fever) may occur in VZV infection approximately one week after rash.
Therapy
Patients with herpetic encephalitis should be treated and observed in the ICU. Base is:
- Thorough monitoring of vital functions.
- Early detection of convulsion (spasm) activity - seizures are very frequent during herpes encephalitis. We initiate anticonvulsant treatment without a clinical correlate if there is a specific finding on EEG. Commonly used benzodiazepines usually stop the spasm, but due to their short action they do not prevent recurrent seizures. Then a continuous administration of midazolam (eg) or a drug with a longer duration of action (barbiturates) is necessary.
- Early detection of an increase in intracranial pressure.
We provide patients with adequate nutrition and hydration according to their abilities (parenteral, nasogastric tube, orally). In case of proven intracranial hypertension, we initiate all necessary measures.
Causal medicine are antiviral drugs:
- acyclovir, (clearly preferred for higher efficacy and lower toxicity), at a dose of 20 mg/kg for adult i.v. every 8 hours in infusion for 21 days. Its side effects are minimal - renal dysfunction is described at high doses (therefore we do not administer acyclovir bolus).
- vidarabine
We should start acyclovir immediately after the possibility of herpetic encephalitis is suspected, we never wait with treatment for the confirmation of the infection. Afterwards, if HSV infection is not confirmed, acyclovir may be discontinued. Mortality was 60-70% before acyclovir treatment and 30% after it.
- In all neonates at the end of acyclovir treatment, we perform control lumbar puncture and re-examine HSV PCR. Its negativity is a requirement for the end of therapy. Subsequently, we switch to valaciclovir treatment in p.o. form for months to years. Valaciclovir (Valtrex) after p.o. administration converts rapidly to acyclovir. It is more expensive, but compared to p.o. acyclovir has better bioavailability.
- Adequate rehabilitation is also a part of comprehensive treatment.
Complications
The most common consequences in surviving patients are:
- motor deficits;
- secondary epilepsy syndrome;
- change in mental state.
The consequences are more frequent in patients with delayed treatment. Patients who started treatment within 5 days of the first symptoms have better results. Interestingly, both HSV-1 and HSV-2 have comparable mortality, but HSV-2 has higher morbidity, ie more frequent neurological consequences (motor deficits, convulsions, microcephaly, sight defects). Subsequent psychiatric changes (hypomania, various degrees of amnesia, Klüver-Bucy syndrome), recurrent aseptic meningitis - Mollaret's meningitis are also reported in connection with herpes encephalitis.
Mollaret's meningitis
It is a recurrent benign aseptic meningitis. Headaches, meningeal symptoms and fever are typical, and neurological symptoms are only transient. In the cerebrospinal fluid we find mixed pleiocytosis with mononuclear cells, polynuclear cells, endothelial cells (Mollaret cells) and elevated gammaglobulins. PCR can detect HSV-2, uncommonly also EBV or enteroviruses. The therapy is only symptomatic, as the symptomatology disappears spontaneously within a few days. Prophylactic administration of acyclovir or valaciclovir may prevent attacks.
Links
Source
- MUDr.HAVRÁNEK, Jiří: Herpetická meningoencefalitida
Similar articles
Herpes zoster
Varicella zoster virus (VZV) causes clinically 2 different diseases:
- Chickenpox (Varicella)
- Shingles
VZV causes common encephalitis, rarely acute disseminated encephalitis.
Patogenesis
The virus survives in the latent form in the sensitive ganglia of the posterior spinal roots: ganglion Gasseri (nervus trigeminus) and ganglion geniculi. The infection ignites when the body is weakened (surgery, general illness, etc.). The infection usually spreads to the periphery in the respective skin dermatome.
Clinical findings
During the dermatome, pain appears, sometimes preceded by itching. In a few days, a serous (even partially hemorrhagic) rash of various magnitudes appears, and the regional lymph nodes are painful and swollen. The blisters dry out after 2 weeks, leaving depigmentation or hyperpigmentation, the pain gradually disappears. In some patients, severe neuralgic pain persists in the respective dermatome even after the crust separates. Paresis are present in 20% of the cases, encephalitis is rare.
- Herpes zoster oticus
When the ganglion geniculi is affected. The blisters appear in the ear canal and around the ear itself. Hunt's syndrome - hearing impairment, dizziness, paresis of the facial nerve (n. VII).
- Herpes zoster ophthalmicus
Gasseri ganglion lesion. Sowing of blisters in the first branch of the trigeminal nerve (ophthalmic nerve). It causes conjunctivitis and the risk of keratitis. 50-70% of patients have eye complications. It occurs predominantly at an older age (in the 5th-8th decade). We find an increase of lymphocytes in the cerebrospinal fluid.
Treatment
The drug of choice is aciclovir. Descending treatment of prednisone. Also liquid powder for the treatment of the blisters, ATB against superinfection and for neuralgia carbamazepine or tricyclic antidepressants.
Vaccination
Zostavax live vaccine is indicated for people over 50 years of age. After undergoing shingles, an interval of 6 months is recommended. Contraindicated in pregnant, immunosuppressed, active untreated TB, hypersensitivity to vaccine components. Available in the Czech Republic since 2014, it is not covered by health insurance.
Links
References
- SEIDL, Zdeněk a Jiří OBENBERGER. Neurologie pro studium i praxi. 1. vydání. Praha : Grada Publishing, 2004. ISBN 80-247-0623-7.
- ↑ Medixa - Herpes zoster
- ↑ TOPINKOVÁ, Eva. Herpes zoster a očkování proti němu [online]. www.ProLékaře.cz, ©2014. [cit. 30. 10. 2014]. <https://www.prolekare.cz/preventabilni-onemocneni-novinky/herpes-zoster-a-ockovani-proti-nemu-4256,>.
Source
Rabies (rabies, lyssa)
Rabies (lyssa) is a viral infectious disease that spreads from wild and domestic animals. Once clinical symptoms break out, the disease is always fatal.
The Czech Republic has been considered "Rabies-free" since 2004 (rabies does not occur in the Czech Republic). In the Czech Republic, you can find imported rabies or rabies after a bat bite. There is an increased risk of rabies at the borders with Poland and Slovakia (these countries are not "Rabies-free").
Pathogenesis
- Primary encephalitis caused by lyssavirus belonging to the Rhabdoviridae group,
- transmitted with the saliva of the animal when bitten or infected by scratches,
- the virus spreads perineurally from the site of contamination to the CNS,
- the length of the incubation is determined by the distance of the injury from the head (2 weeks to several months).
Pathological-anatomical image
- In the cytoplasm of infected cells, inclusion Negri bodies,
- The virus attacks the cerebral cortex, oblongatum, nuclei of the cranial nerves and spinal ganglia.
Clinical signs
- After incubation, non-specific symptoms appear - increased fatigue, emotional lability, sleep disorders, scar tension,
- later paroxysms convulsions - pharyngospasm with dysphagia, hydrophobia,
- profuse salivation and sweating,
- death in a few days due to symptoms of hyperpyrexia and heart failure.
Differential diagnostics
It is necessary to differentiate tetanus differentially.
Treatment
There is no cure for rabies.
Prophylaxis
It is necessary to have the animal examined by a veterinarian. When bitten by an infected animal or an animal at risk of rabies, so-called post-exposure prophylaxis is necessary. It is a combination of active (vaccine) and passive (antirabies heterologous horse serum) immunization. The vaccine is given in 5 doses on a schedule of 0-3-7-14-28. Pre-exposure prophylaxis is also possible for travelers and veterinarians.
Reye's syndrome
Poliomyelitis
Poliomyelitis (also referred to as polio) is an infection caused by the poliovirus, a virus of the Enterovirus genus, family Picornaviridae. Poliovirus is a single-stranded RNA virus with a protein capsid. In its most acute cases, it selectively destroys lower motor neurons of the spinal cord and brainstem which results in unilateral flaccid weakness or paralysis of the limbs. It may also cause death due to acute respiratory arrest. The illness can be characterized as a "remembered" disease, since it has been largely eradicated throughout the second half of the 20th century; however, the World Health Organization estimates there are still twenty to thirty million survivors alive. The global polio pandemic of the 1950's has had historical ramifications on the course of medicine, since it spurred the creation of the first Intensive Care Units and launched the first mass inoculation campaigns.
Epidemiology
Poliovirus, like all enteroviruses, is ingested through contaminated water or foods, proliferates in the gastrointestinal tract, and is then shed in the feces of the infected individual. It is, therefore, transmitted through the "fecal-oral route". The virus enters through the mouth and nose, multiplies in the throat and intestinal tract, and then is absorbed and spread through the blood and lymph system. The incubation time ranges from 5 - 35 days (average 7 - 14 days). In the United States, cases of wild-type poliovirus infections have not been reported in more than twenty years. The few cases that do occur are caused by the reversion to virulence in the live-attenuated Sabin polio vaccine. Wild-type poliovirus has been eliminated from Western Europe, Japan, and the Americas. However, it is still endemic in sub-Saharan Africa and southern Asia.
Pathogenesis
Poliovirus may follow one of several courses:
- asymptomatic infection (in 90-95% of cases);
- abortive infection;
- non-paralytic infection;
- paralytic poliomyelitis (in about 1% of cases).
The mechanism of its spread from the alimentary tract to other systems (e.g. CNS) has not yet been substantiated. However, in all cases of spread, a primary viremia is present after infection. The theories of spread include infection through infected monocytes crossing the blood-brain barrier, retrograde axonal infection of neurons contacting infected tissues in the periphery and transmitting it to the central nervous system, or simple direct passage of the virions through the blood-brain barrier.
Symptoms
Infections of the poliovirus have three patterns of infection. These are subclinical symptoms which may not be present or may only last for less than 72 hours), and clinical patterns which are further divided into non-paralytic and paralytic poliomyelitis. The symptoms for each of the patters are:
Subclinical Infection Symptoms
Symptoms may be absent, or gone after first 72 hours. They include malaise, headache, sore throat, slight fever, vomiting.
Non-paralytic poliomyelitis
Symptoms include back pain or backache, diarrhea, excessive tiredness, fatigue, headache, irritability, leg pain (calf muscles), moderate fever, muscle stiffness, muscle tenderness and spasm in any area of the body, neck pain and stiffness, pain in front part of neck, pain or stiffness of the back, arms, legs, abdomen, skin rash or lesion with pain, vomiting.
Symptoms last 1-2 weeks.
Paralytic poliomyelitis
Usually starts with a fever 5-7 days before the appearance of other symptoms. Symptoms include abnormal sensations (but not loss of sensation) in an area, bloated feeling in abdomen, breathing difficulty, constipation, difficulty beginning to urinate, drooling, headache, irritability or poor temper control. Muscular complications include muscle contractions or muscle spasms in the calf, neck, or back, muscle pain, muscle weakness that is only on one side or worse on one side. The location of this pain depends on where the spinal cord is affected. This pain worsens into paralysis, and sensitivity to touch.
Postpoliomyelitis syndrome
About twenty to thirty five percent of patients who recover from paralytic poliomyelitis have new onsets of muscle weakness, pain, atrophy and fatigue 25 to 35 years after the onset of the acute infection.
Prognosis
Permanent weakness is observed in about two out of three patients affected with paralytic poliomyelitis. Patients who require mechanical ventilation (known as the iron lung in the pandemic era) rarely recover without some form of permanent disability.
Treatment, and prevention
No specific antiviral treatment is yet known for poliovirus, so treatment remains supportive and symptomatic. During the 1950's global pandemic, mechanical respiratory assistance was given through the iron lung. Vaccinations, therefore remain to be the most effective way for dealing with the disease.
Vaccines
The polio vaccine was developed by Jonas Salk in 1952, and introduced to the world in 1955. The oral vaccine was developed by Albert Sabin and licensed in the early 1960’s. The discovery of these vaccines was critical to the eradication of this disease due to the fact that immunocompetent individuals do not have a long-term carrier state, the fact that polioviruses do not have a non-primate reservoir in nature, and that survival of the virus outside of a host is practically non-existent. Therefore, by providing the vaccine and the subsequent human-to-human transmission, poliomyelitis could be a key step in the disease’s global eradication.
Salk Vaccine
The Salk vaccine, or inactivated poliovirus vaccine (IPV) is based on strains of the three known serotypes of the virus, and is cultured as a Vero cell line tissue culture (derived from monkey kidney tissue). The vaccine induces an IgG-mediated immunity in the blood, preventing polio from progressing to viremia (an obligatory condition to be met for the further infection of the host). The strains are then inactivated with formalin.
Oral Poliovirus Vaccine
The oral poliovirus vaccine (OPV) is the form of the vaccine used mostly during the eradication campaigns by the WHO and allied groups. This version of the vaccine is produced through passage of the virus through non-human cells at a sub-physiological temperature. This produces spontaneous mutations to the viral genome. Eventually, 57 nucleotide substitutions distinguish the viral strains found in the OPV from the Mahoney serotype. The other serotypes are also present in the OPV with similar mutation rates. The location of these mutations is a key factor to the attenuation of the virus: The mutations are found in the IRES, thus interfering with the ability of the virus to translate its RNA genome in the host cell. The strains found in the Sabin OPV are able to efficiently reproduce in the gut, however they are not able to do so in nervous tissue, thus making it safe for the recipient. Apart from its high effectiveness, the other major reason for the OPV to be chosen for wide distribution in mass vaccination campaigns is the elimination of the need for sterile syringes.
The Salk (killed polio) vaccine has no adverse effects, whereas the OPV may undergo reversion to a virulent form while it multiplies in the GIT and propagate to cause paralytic poliomyelitis. Although the wild-type poliovirus has been eradicated in Western Europe, Japan and the Americas.
Acquired Immune Deficiency Syndrome (AIDS)
The disease, known since 1981 [1] is caused by the HIV retrovirus. Neuro- and lymphotropic properties allow the virus to invade the CNS, as well as progressively destroy the immune system (destroying T4 lymphocytes). It is transmitted horizontally (sexual intercourse, blood derivatives, parenteral drug use) or vertically (mother-to-child). Groups that are more at risk of infection are, for example, homosexuals, drug addicts, hemophiliacs or children of infected mothers.
Clinical signs
Acute infection is indicated by fever and numbness of the nodes. Anti-HIV antibodies do not appear until 1-3[1] months at the earliest, and their positivity is the only manifestation in up to 70% of those infected[1]. This stage of symptomatic chronic infection can last for months to years. Patients often have symptoms we call the AIDS-related complex (ARC), which includes diarrhea, lethargy, weight loss, opportunistic infections (candidiasis, impetigo). HIV positivity is also accompanied by lymphopenia, thrombocytopenia. AIDS means progression to severe opportunistic infections and tumors, such as:
- pneumocystis pneumonia (50%),
- Kaposi's sarcoma (20%), CNS lymphoma,
- non-Hodgkin's lymphomas,
- tuberculosis.
Neurological manifestations occur in 80% of patients[1], their onset is related to seroconversion and late AIDS.
Treatment
Treatment should be started immediately, if cerebral toxoplasmosis is suspected, we administer pyrimethamine or sulfadiazine. If the CT image does not improve, we indicate a biopsy (lymphoma). AIDS inevitably ends in a fatal infection or malignancy. Drugs that mobilize the immune system and affect the replication of the virus, such as zidovudine (AZT), which blocks the production of HIV-specific DNA and thus reduces the replication of the virus, are also being tested. We also use immunomodulators in therapy, such as IFN, IL-2.
Subacute sclerosing panencephalitis
Subacute sclerosing panencephalitis is a rare fatal disease in children and adolescents that usually begins between 7 and 10. year of age, typically 6-7 years after measles. It hardly occurs in countries with high vaccination coverage.
Etiology and pathogenesis
It is a slow viral disease in which a mutant form of measles paramyxovirus attacks the central nervous system. In patients, there is a change in the reactivity of the immune system against the measles virus, so it is not an immune disorder as such. Measles infection before the second year of age, when the immune system is maturing, is a big risk. In pathogenesis, there is a secondary disorder of the immune system.
Vaccinations are sometimes mentioned in the literature. Such a hypothesis was there, but it failed to confirm it and is currently considered safely invalid.
Epidemiology
The incidence of subacute sclerosing panencephalitis varies geographically, with an estimated 4–11 per 100,000 patients in the United States in the 1960s. It was more common in children under the age of five, where it reached a frequency of 18 per 100,000 patients. In the Middle East, the situation is more critical, with children under one year of age suffering from sclerosing panencephalitis with a frequency of up to 360 per 100,000. An analysis of cases in Germany from 2003 to 2009 was published in 2013. A total of 31 cases were reported, of which subacute sclerosing panencephalida is estimated to affect 30 to 59 out of 100,000 children who experience measles before the age of five. In 2016, an analysis of cases of subacute sclerosing panencephalitis in California in 1998-2016 was published. 17 cases have been caught, with an estimated frequency of 73 per 100,000 diseases for children who have had measles before the age of five, and 164 per 100,000 for children under one year of age.
Clinical signs
At first, it manifests as inconspicuous behavioral and concentration disorders that result in dementia . Later, myoclonus , choreatic movements and epileptic seizures appear. Increasing spasticity is also common.
Diagnostics
Subacute sclerosing panencephaitis is diagnosed by symptoms and test results, such as typical changes in EEG, elevated measles gamma globulins in cerebrospinal fluid and serum. At the beginning, typical R-complexes (Rademakers) are seen on the EEG as multiple spikes and slow waves. This is followed by a gradual breakdown of the record and the cessation of the activity.
The course
As many as 80% of children die within 3 years of the event and in 10% the fulminant course ends lethal within 3 months. The remaining 10% die in 4-10 years.
Therapy
The prognosis can be affected only minimally. Although experience with the inosiplex immunomodulator is promising, it is obtained on only a relatively small sample of patients. . The basis of treatment is symptomatic therapy, ie antiepileptics . The introduction of measles vaccination has significantly reduced the incidence of this disease, eg only 4-5 cases are currently reported in the USA.
Progressive multifocal leukoencephalopathy
It is a rare viral infection caused by demyelination of nerves. The condition is a failure of cellular immunity. It is a fatal complication of diseases such as lymphoma, leukemia, SLE, HIV infection and sarcoidosis.
Etiology
Progressive multifocal leukoencephalopathy (PML) is caused by reactivation of saprophytic papovavir.
Clinical picture
Papovirus attacks oligodendroglia and causes demyelination of nerves. This process takes place without any signs of inflammation. Important clinical symptoms include:
- personality change,
- cortical blindness,
- seizures,
- progressive dementia,
- hemiparesis.
Patients may have extensive diffuse hemispheric involvement and death occurs without remission in 3-6 months.
Diagnosis
Progressive multifocal leukoencephalopathy is in most cases diagnosed at an advanced stage. Brain biopsy, CT and MRI. In these examinations, we observe multi-focal changes in white matter. In the cerebrospinal fluid we find an increase in gamma globulins.
Therapy
There is no therapy for this disease yet, with half of the patients dying within a few months of diagnosis. [1]
Portal: Neurology
Portal: Microbiology
Portal: Infectious medicine
Links
Related articles
References
- ↑ a b c d e f Cite error: Invalid
<ref>tag; name "Seidel" defined multiple times with different content - ↑ a b VOKSHOOR, A a C WAN. https://emedicine.medscape.com/ [online]. ©2007. [cit. 2.7.2009]. <https://emedicine.medscape.com//article/1168529-overview>.
- ↑ SEIDL, Zdeněk a Jiří OBENBERGER. Neurologie pro studium i praxi. 1. vydání. Praha : Grada Publishing, 2004. ISBN 80-247-0623-7.
- ↑ SEIDL, Zdeněk a Jiří OBENBERGER. Neurologie pro studium i praxi. 1. vydání. Praha : Grada Publishing, 2004. ISBN 80-247-0623-7.