Trace elements
Trace elements (micronutrients) are found in tissues at concentrations below 50 ppm (<50 × 10-6 g/g)[1]. The main elements - Fe, I, Cu, Zn, Co, Cr, Mo, Se, F, Mn, Ni, As, Sn, Si, V.
are essential - the body cannot make them on its own and is dependent on their intake from food;
their deficiency or excess causes health problems.
The main biochemical role of trace elements is catalytic action in enzymes and modulation of enzyme activities. They are also important in protection against oxidative stress (SOD needs Mn, Cu and Zn).
In the acute phase reaction, IL-1 and IL-6 cause redistribution of trace elements (decrease in serum Zn and Fe).
Toxicity - there is a large tolerance in the concentration range in healthy individuals. Se and Cr may be less easily excreted in renal disorders, Cu and Mn in liver disease.
They are determined by atomic absorption spectrophotometry.
Iron[edit | edit source]
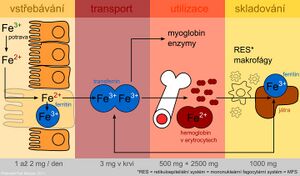
Iron is one of the most important elements in the human body. The adult body contains more than 70 mmol (4.0-4.5 g) of iron. In women, this amount is lower than in men, which is attributed to blood loss during menses.
Iron distribution in the body
| Form | Function | Protein | Quantity in g |
|---|---|---|---|
| Active iron | oxygen transport | hemoglobin | 2.5-3.0 |
| myoglobin | 0.3 | ||
| electron transfer | cytochromes, cytochrome oxidase | 0.2 | |
| cytochromes, cytochrome oxidase | catalase, peroxidase | ||
| Storage iron | ferritin, hemosiderin | 0.8-1.0 | |
| Transport iron | transferrin | 0.003 | |
Iron metabolism[edit | edit source]
The presence of iron is essential for cell function. As a component of heme, it is involved in oxygen transport and as a component of cytochromes, it conditions electron transfer in the respiratory chain. A side effect of iron as a transient and highly reactive element is its participation in radical reactions, which produce so-called reactive oxygen species. These can damage cell membranes, proteins and DNA.
Iron is absorbed as Fe2+ by active transport in the duodenum and upper jejunum in two ways:
- Porphyrin-bound Fe in the form of a stable lipophilic complex;
- FeII+ - water-soluble chelates.
Only a small part is absorbed in ionised form.
On average, 10-50 mg of iron per day is present in the diet, but only 10-15% is absorbed. In heme compounds (meat) it is absorbed better, non-heme Fe in plant food much less well. In addition, plants contain oxalates, phytates, tannins and other phenolic compounds that form insoluble or chelate complexes with Fe that are difficult to absorb. Ascorbic acid, on the other hand, improves iron absorption.
After uptake by the intestinal mucosa, part of the iron is incorporated into a storage form, ferritin, in intestinal cells. Part of the absorbed iron is transferred to the plasma, where it is transported bound to transferrin. An important role in the transfer of iron across the basolateral membrane of enterocytes is played by the protein ferroportin (also found in the membrane of macrophages and hepatocytes). It is the main site of regulation of iron homeostasis in the body. A key factor in regulation is the protein hepcidin, which is synthesized in the liver. By binding to ferroportin, it inhibits the transport of iron out of cells and thus contributes to its sequestration in them. The level of hepcidin increases during inflammation. Hepcidin is partly responsible for the anaemia of chronic diseases. Mutations in the gene for hepcidin lead to juvenile haemochromatosis type 2B.
Plasma iron is taken up by target tissue cells via the transferrin receptor and is either incorporated into heme or stored as ferritin. The use of the specific transferrin transport protein and the iron storage protein ferritin represent protective mechanisms to prevent the toxic effects of oxidoreductively active iron.
During desquamation of dead mucosal cells, unused iron is passed in the faeces along with unabsorbed iron.
Examination of iron metabolism[edit | edit source]
Diseases associated with changes in iron metabolism and utilization are commonly encountered in practice. Laboratory investigation of iron metabolism includes the following tests:
- serum iron
- serum transferrin and iron binding capacity
- serum ferritin
- transferrin receptor
These parameters are important diagnostic indicators for the detection of a decrease or increase in iron stores even at stages that are not accompanied by significant clinical manifestations.
Determination of iron in serum[edit | edit source]
For the determination of iron in serum, colorimetric methods, atomic absorption spectrophotometry and other special techniques are used. Photometric methods, based on the reaction of iron with a complexing agent, are the most widely used. All procedures involve the following steps:
- Release of Fe3+ from its bond to transferrin using acids or tensides (e.g. HCl).
- Reduction of Fe3+ to Fe2+, which is necessary for the reaction with the complexing agent. E.g. ascorbic acid is used for the reduction.
- Reaction of Fe2+ with a complexing agent containing reactive groups -N=C-C=N- to form a coloured complex. The metal ions form chelates with two nitrogen atoms. Currently, two complexing agents are mainly used - bathophenentroline and ferrozine (3-(2-pyridyl)-5,6-bis(4-sulfophenyl)-1,2,4-triazine - PST, protected name FerroZine®), which has a higher absorption coefficient and is more soluble in water.
Rating
Serum iron concentrations are subject to circadian rhythms and are influenced by other factors. This limits the diagnostic significance of this parameter. It is a poor indicator of tissue iron stores and should always be assessed in combination with serum transferrin and iron binding capacity. Decreased concentrations accompany iron deficiency, e.g. due to large or repeated blood losses, inadequate dietary iron intake or impaired absorption. The finding is not specific, as reduced levels are also encountered in acute infection or chronic inflammatory diseases (transfer of iron into tissues). High iron levels occur in haemochromatosis (see below), in iron overdose or intoxication, in increased erythrocyte breakdown and in some liver diseases.
Reference values
men: 9-29 μmol/l
women: 7-28 μmol/l
Serum transferrin and iron binding capacity[edit | edit source]
Iron is transported through the blood by binding to a specific protein with β1-electrophoretic mobility, transferrin, which is synthesized in the liver. The rate of its formation is inversely proportional to the iron stores in the body; it increases with iron deficiency and decreases with excess. The biological function of transferrin lies in its ability to readily form non-toxic complexes with iron and to transfer Fe absorbed by the mucosa of the small intestine to the bone marrow or to storage forms (ferritin or hemosiderin). Each molecule of transferrin binds two Fe3+ atoms (1 g of transferrin binds 25.2 μmol of iron). Transferrin can be determined directly by immunochemical methods or indirectly as the iron-binding capacity of transferrin - the so-called iron-binding capacity. The total iron binding capacity (TIBC) is the amount of iron that transferrin is able to bind if all binding sites are occupied. Usually only 1/3 of the transferrin is saturated with iron - the binding capacity. Free transferrin with no iron bound represents the free binding capacity (2/3 of transferrin) available for iron transport at elevated demands.
Conversion between transferrin concentration and total binding capacity:
Total binding capacity [μmol/l] = transferrin [g/l] - 25.2.
The reference range for serum transferrin concentration (S-transferrin) is 2.0-3.6 g/l and for total binding capacity is 50-70 μmol/l.
Transferrin saturation[edit | edit source]
From the iron and transferrin concentration values, we can calculate the transferrin saturation (TfS), which is defined as the ratio of the serum iron concentration to the total transferrin binding capacity for iron. This is a sensitive parameter for detecting latent iron deficiency.
Assessment of transferrin saturation
- Physiological values: 25-50%
- Reduction in iron deficiency saturation: < 15%
- increase in iron saturation: > 50 %
Ferritin and hemosiderin[edit | edit source]
Ferritin is the most important storage protein for iron. The ferritin molecule is adapted to bind large amounts of Fe3+ in a soluble and non-toxic form for the organism. Ferritin is composed of an outer protein shell of 24 subunits - apoferritin (Mr 440,000), enclosing a cavity in which up to 4500 iron atoms can be concentrated in the form of ferric oxyhydroxide (FeO-OH)n in microcrystalline form with phosphates (FeO-OPO3H2). The entry and exit of iron atoms is facilitated by the pores between the subunits of the ferritin molecule shell. Normally, about 20 % of its capacity is used. It is stored in cells in the liver, spleen and intestinal mucosa.
In blood serum, ferritin is found in very low concentrations. Serum ferritin concentrations are a measure of the body's iron stores. Low concentrations indicate depletion of the total body iron reserve and are used for early detection of iron deficiency anaemia in the prelatent phase. Elevated ferritin concentrations are a concomitant of high tissue iron stores. They are also encountered in many patients with liver disease, some malignant tumours (tumour marker) or inflammatory diseases (positive reactant of the acute phase).
The reference range for serum ferritin concentration (S-ferritin) is 30-300 μg/l for men and 20-120 μg/l for women.
Hemosiderin is another storage protein for iron. It is formed by aggregation of denatured ferritin with other components. It forms particles of 1 to 2 μm in size, which are visible in the light microscope when stained for iron. Hemosiderin contains a greater amount of iron than ferritin but is difficult to obtain due to its poor solubility in water. It is formed when the amount of iron in the body exceeds the storage capacity of ferritin.
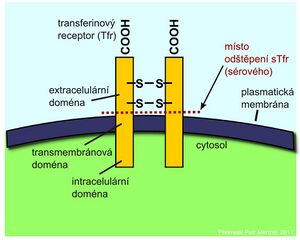
Transferrin receptor[edit | edit source]
Iron transported through the blood by transferrin is taken up by cells via a specific transferrin receptor (TfR). It is found on the surface of all cells at some stage of development, but is expressed most strongly on the surface of red line precursor cells in the bone marrow. TfR is a transmembrane protein that consists of two identical subunits linked by a disulfide bond. Separation of the extracellular domains of the receptor releases the so-called soluble fraction of the transferrin receptor (sTfR) into the circulation, which may be in the form of a dimer or a monomer. Cells respond to a reduction in iron stores by synthesizing increased amounts of transferrin receptor.
An increase in sTfR is a reliable indicator of iron deficiency for hematopoiesis. Elevated sTfR levels are encountered in iron deficiency anemias or in hemolytic anemias. Determination of sTfR is valuable in anemic patients in whom ferritin is elevated because of an acute phase reaction. Determination of sTfR concentration can also be used in bone marrow transplant patients to monitor the progress of erythropoiesis.
Immunochemical methods are used for the determination.
Iron metabolism disorders[edit | edit source]
Iron deficiency (sideropenia)[edit | edit source]
Iron deficiency is usually caused by insufficient absorption of iron from the intestine or chronic blood loss. It can result in sideropenic anaemia (hypochromic microcytic anaemia), which is one of the most common haematological diseases. However, anaemia is usually a late symptom of gradually developing sideropenia. It becomes apparent in the blood count only after almost complete disappearance of iron. Therefore, it is necessary to detect iron deficiency at an early stage that is not yet accompanied by anemia.
On the basis of the determination of basic parameters of iron metabolism, three degrees of deficiency are distinguished:
- Prelatent iron deficiency refers to a condition in which there is a gradual decline in iron stores but iron delivery to the bone marrow erythroblasts is not yet affected. In about half of the patients, serum ferritin levels are reduced below 12 μg/l.
- In latent iron deficiency, iron stores are essentially depleted. Ferritin is reduced below the lower limit of normal and is at this stage already accompanied by a reduction in serum iron levels and reduced delivery to the bone marrow erythroblasts. The binding capacity for iron increases. A sensitive indicator of latent iron deficiency is a decrease in transferrin saturation below 15%. However, anaemia does not yet develop.
- In manifest iron deficiency, anaemia develops with a fall in haemoglobin values below the lower limit of normal. In iron deficiency anemia, the typical finding is low serum iron and ferritin, and the transferrin concentration (iron binding capacity) is elevated. In hemolytic or iron excess anemias, on the other hand, serum iron is elevated and total iron binding capacity is decreased.
Laboratory findings in iron deficiency
| Prelatent iron deficiency | Latent iron deficiency | Manifest iron deficiency |
|---|---|---|
| decrease in iron storage - decrease in ferritin | lack of iron storage - decrease in ferritin | lack of iron storage - decrease in ferritin |
| decrease in serum iron | decrease in serum iron | |
| decrease in transferrin saturation below 15% | decrease in transferrin saturation below 10% | |
| increase in total iron binding capacity | increase in total iron binding capacity | |
| increase in sTfR | increase in sTfR | |
| decrease in haemoglobin concentration - anaemia |
Excess iron[edit | edit source]
The body is not equipped with an excretory pathway for iron and therefore, under certain circumstances, excess iron can accumulate in the tissues. Early diagnosis can prevent tissue damage from excess iron. Iron overload usually develops very slowly. There are 3 stages:
- In the stage of prelatent iron excess, the iron content in the organs increases, but without exceeding their storage capacity.
- In the latent stage of iron overload, cellular storage capacity is exceeded but organ function is not yet impaired, ferritin and serum iron levels increase, and transferrin saturation rises above 55%.
- In the phase of manifest iron excess, some organs are already damaged.
Laboratory findings in iron excess
| Prelatent iron excess | Latent iron excess | Manifest iron excess |
|---|---|---|
| Increase in iron stores - increase in ferritin | Increase in iron stores - increase in ferritin above 300 μg/l | Increase in iron stores - increase in ferritin (in severe disability above 2000 μg/l) |
| increase in serum iron | significant increase in serum iron | |
| increase in transferrin saturation above 55% | increase in transferrin saturation (may exceed 90% in severe disability) |
Haemochromatosis
The accumulation of iron in tissues is related to a disease called haemochromatosis.
- Primary hemochromatosis is a hereditary disease caused by increased resorption of iron from the intestine. Excess iron is stored in parenchymatous organs such as the liver, heart, pancreas, and adrenal glands. In the affected organs it is toxic and impairs their function by catalysing chronic reactions leading to the formation of free radicals. The main clinical manifestations are hyperpigmentation of the skin, hepatosplenomegaly and diabetes mellitus.
- Secondary haemochromatosis may develop as a consequence of, for example, repeated transfusions, excessive intake of iron-containing preparations or haemolytic anaemia. In the biochemical picture, we find increasing levels of ferritin and iron in the serum, increasing transferrin saturation with a concomitant decrease.
Iron poisoning[edit | edit source]
Accidental ingestion of large quantities of preparations threatens children (lozenge-like tablets). The lethal dose for a child is 600 mg. For an adult, an iron intake of 40 mg/kg is toxicologically serious; an intake of 60 mg/kg is fatal.[2]
Symptoms include nausea, vomiting (even vomiting blood), abdominal pain, diarrhea (sometimes bloody). Large fluid losses cause shock, renal failure and death. If the patient survives this stage of poisoning, he may fall unconscious, convulse and go into liver failure after 12 hours. If he or she survives this second phase, the poisoning may leave permanent effects (damage to the intestine).
Treatment of acute poisoning
- Gastric lavage.
- Nasogastric probe to administer the chelating agent deferoxamineState Drug Control Authority: deferoxamine (5-10 g in 50-100 ml of water).
- Consider intravenous administration of desferoxanine to balance absorbed iron. A pinkish-red complex of deferoxamine with iron appears in the urine. Treatment should be repeated until the urine colour returns to normal.[2]
Iodine[edit | edit source]
Iodine is involved in the production of thyroid hormones.
Source[edit | edit source]
Sources of iodine are mainly marine fish and their products and iodized salt. In the Czech Republic, salt is iodized at 20-34 mg/kg of salt in the form of iodide or iodate. The iodine content of plant and animal foods depends on the iodine content of the soil and its supply to livestock. Milk and eggs tend to be good sources of iodine. Some iodine is lost through cooking.
The recommended daily intake for adults is between 150 and 200 μg (varies by country and age).[1]
Stromogens, which are antinutritional substances, may also contribute to iodine deficiency disease. Strumigens can be divided according to their mode of action:
- Tier I strumigens - prevent iodine fixation (thiocyanide, nitrates, polysulfides from cabbage).
- Tier II Strumigens - block peroxidases that convert iodine (radishes, onions, peas, tomatoes, spinach).
- Tier III Strumigens - block the production of thyroxine (sulfonamides).
- Tier IV Strumigens - competitively displace thyroxine, inhibit TRH secretion.
Iodine deficiency[edit | edit source]
Manifestations that can be prevented by sufficient iodine intake are referred to by the WHO as iodine deficiency disorders. (Iodine deficiency disorders - IDD). This term was chosen to emphasize that the problem is far broader than mere goiter and cretinism, the classic and most visible symptoms of iodine deficiency. The spectrum of iodine deficiency disorders varies in severity and by age. These include milder impairments of mental function, delayed physical development, reduced fertility, increased stillbirths and perinatal mortality.[3]
The most critical period is from the 2nd trimester of pregnancy to the end of the 3rd year of life. Normal levels of thyroid hormones are required for brain development to occur for proper myelination of axons. In iodine-deficient areas where thyroid hormone levels are low, brain development is impaired. The most severe impairment results in the development of cretinism, but at the population level, less severe degrees of impairment and reduced cognitive capacity are far more severe and affect the entire population. As a result, the mental abilities of "normal" children and adults living in iodine-deficient areas are lower than those in non-deficient areas - by as much as 13.5 points on the IQ scale.[3]
Worldwide, iodine deficiency disorders are among the most prevalent malnutrition disorders.
Assessing the iodine supply status of the population[edit | edit source]
Until the 1990s, the prevalence of goiter was used as the primary indicator of iodine deficiency in the population. Later, urinary iodine excretion, a sensitive indicator of recent iodine intake, became the method of choice for assessing and monitoring the iodine status of the population. If more detailed information is needed, TSH levels are measured. Plasma TSH and thyroid hormone concentrations: TSH and plasma triiodothyronine (T3) typically increase in deficiency, while plasma thyroxine (T4) decreases. However, these changes may only become apparent in severe deficiency. School children are most commonly evaluated, and their status usually reflects that of the general population.[3]
Excess[edit | edit source]
Iodine excess-induced goiter, thyrotoxic crisis or acne have been described after exceeding the recommended dose of iodine intake by several orders of magnitude. Even chronically high iodine intake, e.g. from iodine-containing drugs or disinfectants, can lead to the development of goiter.
The EU tolerable upper limit of long-term iodine intake for adults is 600 μg/day.[4]
Zinc[edit | edit source]
Zinc is required for the activity of more than 200 Zn-dependent metalloenzymes (carbonic anhydrase, alcohol dehydrogenase, LDH, ALP, superoxide dismutase, etc.), necessary for DNA synthesis and for the function of some proteins that bind to DNA (see zinc fingers) → deficiency affects growth, healing, deficiency in the fetus causes e.g. spina bifida. Zinc is a component of superoxide dismutase, forms a structural and functional component of biological membranes, stabilizes the structures of RNA, DNA and ribosomes. Zn is necessary for cell proliferation, cellular immune reactions, stabilization of the hormone-receptor complex. It is necessary for the proper function of male gonads.
Source[edit | edit source]
Zinc is abundant in meat and other protein-rich foods, whole grains, legumes, root vegetables. It is better utilized from animal than from plant foods, from which it is less easily absorbed because of its phytate, fiber and some other substances.[5]
The absorption of zinc depends on its intake and the body's zinc supply - at high intake, absorption decreases and excretion into the intestine increases, and vice versa, at low intake, absorption increases and excretion into the intestine decreases. Unlike Cu and Fe, Zn is not stored in the liver. About 10 % is excreted in the urine, the remainder is taken up in the bile via pancreatic secretions.
The recommended daily dose for adult males is 10 mg and for females 7 mg.[1]
Deficiency[edit | edit source]
Zn deficiency may be genetic or acquired primarily (i.e. through inadequate diet) or secondarily (i.e. conditioned by a disease leading to, e.g., inadequate absorption).
Zinc deficiency can be caused by insufficient intake. Zinc is also poorly absorbed from foods rich in phytate, fibre and other substances, as these substances inhibit its utilisation. Losses during diarrhoeal diseases, malabsorption syndromes and parasitic diseases may also contribute to zinc deficiency. Zn deficiency is one of the most prevalent malnutrition in the world.
To assess the zinc supply status of a population, the most widely used method is to determine the plasma zinc concentration. However, this indicator can be influenced by a number of factors. A concentration of 10 μ/l is considered sufficient.
Symptoms of deficiency[6]
Acrodermatis enteropathica[7] (Danbolt's disease) is a genetic disorder of Zn absorption, probably due to a defect in the ligand that normally facilitates Zn absorption in the intestine. It develops after birth, shortly after the transition to artificial nutrition. Clinical manifestations are progressive bullous-pustular dermatitis in association with paronychia and generalized alopecia; ocular signs (blepharitis, photophobia and corneal opacity) are often present. Gastrointestinal symptoms include chronic diarrhoea, malabsorption, steatorrhea, lactose intolerance. There are also neuropsychiatric symptoms, delayed growth, hypogonadism and increased susceptibility to infections. Biochemical findings show decreased resorption of Zn in the intestine but normal excretion in faeces, urine and sweat. There is a marked decrease in plasma Zn. Therapeutically, administration of diiodohydroxyquinoline, which forms a well absorbed complex with Zn, is effective.
Even a mild zinc deficiency leads to impaired immunity and increased morbidity and mortality from infectious diseases. It is also manifested by growth retardation, failure to thrive, hypogeusia (impaired taste) and possibly thymic atrophy. Zinc deficiency is common in the elderly, especially the hospitalised, and may manifest itself in poor wound healing.
Significant zinc deficiency was described in 1961 in young men in Iran and was manifested by growth retardation, hypogonadism, delayed sexual maturation, hepatosplenomegaly and anaemia.
Significant zinc deficiency can also occur in patients on parenteral nutrition without zinc and is manifested by alopecia, diarrhoea, skin lesions and anorexia. If zinc is not given, patients die from intercurrent infection (e.g. diarrhoea).
In pregnant women, significant zinc deficiency is associated with birth defects in their babies and spontaneous abortions. Moderate deficiency is associated with fetal growth retardation, low birth weight and birth complications.
Maternal and early infant zinc deficiency can have a negative effect on the neuromotor development of the child.
Treatment with zinc[edit | edit source]
Contraindications to zinc treatment - autoimmune diseases, kidney disorders, pregnancy, lactation.
Toxicity[edit | edit source]
Acute Zn poisoning causes diarrhea, vomiting, nausea, muscle pain and fever. May be caused iatrogenically (infusion, contamination during haemodialysis). Oral administration of Zn may lead to gastrointestinal discomfort, ulceration of gastric mucosa. High doses of Zn can cause Cu deficiency because Zn competes with Cu during absorption in the intestine. This can lead to anemia from Cu deficiency.
Poisoning:
- Occupational diseases - fever from zinc vapour in metal founders - metallic taste in mouth, irritating cough, muscle aches
- respiratory irritation, bronchopneumonia to pulmonary oedema
- skin ulceration
- after ingestion - lethargy
- the decrease in plasma levels may not be due to a decrease in body stores
Copper[edit | edit source]
Copper is essential for the proper function of every cell in the body, the main function is in hematopoiesis (ceruloplasmin), ceruloplasmin oxidase activity in plasma is essential for the oxidation of Fe2+ to Fe3+ → mobilization of Fe and incorporation into heme.
- It is a component of respiratory and antioxidant enzymes;
- Important in the formation of hair and pigments;
- is important for the proper course of immune reactions;
- Lysyl oxidase is required for the cross-linking of collagen and elastin.
Copper is resorbed, bound to albumin and incorporated into ceruloplasmin in the liver. Main excretion - bile. Nutritional deficiency is rare, rather as part of malnutrition. The function of copper is closely related to that of zinc (optimal ratio Zn:Cu = 7:1, when both trace elements act synergistically). Sources of copper are eggs, meat, legumes. The recommended daily dose of copper is 2-2.5 mg.
Symptoms of deficiency[edit | edit source]
Acquired deficiency[edit | edit source]
- Microcytic, hypochromic anemia, leukopenia, osteoporosis;
- Anemia unresponsive to Fe administration, decreased ceruloplasmin;
- More sensitive indicator - decrease in Zn, Cu-SOD activity in erythrocytes;
- immune disorders;
- hair and nail growth disorders.
Congenital deficiency[edit | edit source]
Menkes disease (trichopoliodystrophy, "kinky-hair" syndrome) is an X chromosome-linked inherited disease caused by mutation of the gene encoding the Cu2+-transporting ATPase. This leads to the inability of intestinal mucosal cells to transport Cu across the serous membrane into the blood circulation. It manifests in male infants in the first few weeks; those affected usually die within three years after birth. The disease is characterized by severely delayed mental development and growth, peculiar hair appearance (tiny curls on short fine grey hair - "kinks" or "steely" hair), scorbutic changes on the bones, cerebral gliosis with cystic degeneration, thermal instability and arterial tortuosity (tortuosity). Clinical signs are due to decreased activity of Cu-containing enzymes such as ceruloplasmin, cytochrome c oxidase, superoxide dismutase, lysyl oxidase, dopamine-β-hydroxylase (DBH). The biochemical findings include a marked decrease in plasma copper, a decrease in S-ceruloplasmin, and a decrease in tissue and hair Cu content, with the exception of the duodenal mucosa, which contains abnormally elevated amounts of Cu. Anemia, usually hypochromic and normocytic, is neutropenia; there is also osteoporosis and bone fractures, irregularities in metaphyseal formation. The reduced activity of Cu-metaloenzymes probably plays a major role in the pathogenetic mechanism of the disease: lysyl oxidase (impaired biosynthesis of collagen and elastin leads to changes in bone and vascular wall), as well as cytochrome c oxidase, dopamine-β-hydroxylase and superoxide dismutase, leading to neuronal degeneration and demyelination of brain tissue. Parenteral administration of Cu (immediately after birth) can prevent severe changes. The reduction of DBH activity also causes different concentrations of catecholamines in plasma and cerebrospinal fluid: high levels of DOPA, DOPAC and dopamine, low levels of dihydroxyphenylglycol (DHPG). An elevated DOPA/DHPG and DOPAC/DHPG index is a useful diagnostic marker of Menkes disease.
Toxicity and disease[edit | edit source]
- Copper is relatively toxic when inhaled - "metal fever" - as with Zn;;
- in serum - icterus, liver and kidney damage, often fatal;
- Wilson's disease .
Limited incorporation of Cu into ceruloplasmin and limited excretion by the liver.
Accumulation of Cu in liver, unbound Cu rises, more goes out through kidneys, deposited - in cornea (Kayser-Fleischer ring), in brain (mainly basal ganglia).
Symptoms resemble cirrhosis, there is also rigidity, tremor.
Selenium[edit | edit source]
Se-containing enzymes are very important antioxidants (glutathione peroxidase, phospholipid hydrogen peroxide-glutathione peroxidase), they act in thyroid hormone metabolism.
Source[edit | edit source]
Sources of selenium are cereals grown on selenium-rich soils, marine products. The recommended daily dose of selenium is: 1 mg/kg; 50-200 μg/day. It has a positive effect on the immune system (with selenium deficiency, lymphocyte stimulation decreases, NK-cell activity decreases, interferon production decreases).
It has anticarcinogenic effects (it is part of the antioxidant enzyme glutathione peroxidase, which is involved in the prevention of damage caused by free oxygen and peroxide radicals, see oxidative stress). Promotes sperm maturation and motility.
Selenium is absorbed in the duodenum, reducing its supply of fiber, methionine, Zn and Cd. It is resorbed independently of its content in the body and excreted mainly by the kidneys. It is not stored in the liver; its serum level decreases very rapidly when supply is inadequate.
Deficiency[edit | edit source]
Se deficiency is very common in our country.
Symptoms of deficiency include cardiomyopathy, higher incidence of cardiovascular disease, impaired immunity, increased risk of cancer, myopathy, etc.; selenium deficiency can result in Keshan disease, a juvenile endemic cardiomyopathy described in some areas of China. It is a multi-organ cardiomyopathy affecting mainly young children in areas with Se deficiency in soil and drinking water. In addition to muscle stiffness, weakness and pain, there is depigmentation of hair, skin and nails, and weakening of these tissues.
Long-term parenteral nutrition without Se can lead to fatal cardiomyopathy. Se levels are usually very low long before clinical symptoms appear.
Pregnancy levels tend to be low because the fetus accumulates Se.
Toxicity[edit | edit source]
Se toxicity is rare:
- Acute poisoning is manifested by a garlicky odour from the mouth and sweat due to the presence of dimethyl selenide.
- Chronic selenosis is manifested by hair and nail loss, skin blistering, damage to the dentition.
Chromium[edit | edit source]
Cr3+ is biologically active, Cr6+ is toxic
Functions[edit | edit source]
The trivalent form of chromium is claimed to be a glucose tolerance factor. It stimulates the action of insulin and increases glucose tolerance. In healthy people, it increases HDL levels.
Conversely, occupational exposure to hexavalent chromium has allergenic effects and is carcinogenic.
Source[edit | edit source]
Sources of chromium may include:
- Yeast (brewer's yeast);
- Meat;
- cheese, wheat germ and nuts.
Recommended daily requirement[edit | edit source]
The recommended daily intake of chromium is 150-200 μg.
Deficiency[edit | edit source]
Chromium deficiency can result in:
- Reduced glucose tolerance to type 2 diabetes mellitus;
- hyperlipidaemia;
- acceleration of atherosclerotic changes.
Toxicity[edit | edit source]
Toxic mainly hexavalent - readily crosses membranes and crosslinks DNA → DNA-DNA crosslinks - contributing to mutagenesis. It enters the body from air emissions and damages the respiratory tract, conjunctiva, kidneys.
Manganese[edit | edit source]
Manganese is important for bone structure, CNS function and a number of enzymes (pyruvate carboxylase, SOD, kinases, decarboxylase...).
Functions[edit | edit source]
Involved in the process of oxidative phosphorylation, thus interfering with fat metabolism → highest concentration in cells - in mitochondria.
Source[edit | edit source]
Sources of manganese are oatmeal, whole wheat bread, tea and cocoa. Estimated daily requirement (recommended dose cannot be determined) is 2-3 mg.[8] It is excreted in the bile.
Deficiency[edit | edit source]
Deficiency is exceptional, may cause elevated blood lipid levels and associated premature atherosclerosis. Dermatitis or digestive disorders may also occur.
Toxicity[edit | edit source]
In the 19th century, it was shown in miners - "manganese madness" - psychiatric disorders, parkinsonism.
Molybden[edit | edit source]
- An element of flavoenzymes (xanthine oxidase - purine metabolism).
- The estimated daily requirement (recommended dose cannot be determined) for adults is 50-100 μg. [1]
- Deficiency - methionine rises, uric acid decreases, urinary sulfate low; not present from food - so far described only after prolonged parenteral nutrition[1].
- Toxicity - not very toxic.
Cobalt[edit | edit source]
- A component of B12, it is mainly in leafy vegetables, in the liver;
- Has direct role in EPO formation, inhibits oxidation in bone marrow → after Co administration - polycythemia;
- Deficiency symptoms - anemia, weight loss, fatigue, lack of appetite, growth retardation.
- Toxicity
- polycythemia, thyroid hyperplasia, congestive heart failure;
- Co was formerly put in beer because of foaming - after prolonged consumption of CMP.
Fluorine[edit | edit source]
- Tooth formation, cardiostatic, importance for bone metabolism;
- Dietary sources are marine fish, black tea;
- Adequate daily intake (recommended dose not specified) for adults is 3.2 mg for men and 3.1 mg for women[9]
- Fluoride deficiency increases tooth decay and affects the process of calcium deposition in bones.
- Excess:
- Acute intoxication - spasms, cramps, salivation, sweating.
- Chronic intoxication - mottled teeth, osteophytes, calcification.
Links[edit | edit source]
Related articles[edit | edit source]
- Types of food
- Fats in diet
- Carbohydrates in diet
- Dietary protein
- Minerals in diet
- Nutrition recommendations
Literature used[edit | edit source]
- BENCKO, Vladimír, et al. Hygiena – učební texty k seminářům a praktickým cvičením. 2. vydání. Praha : Univerzita Karlova, 2002. 204 s. ISBN 80-7184-551-5.
- SCHNEIDERKA, Petr, et al. Kapitoly z klinické biochemie. 2. vydání. Praha : Karolinum, 2004. ISBN 80-246-0678-X.
- MASOPUST, Jaroslav a Richard PRŮŠA. Patobiochemie metabolických drah. 2. vydání. Univerzita Karlova, 2004. 208 s.
References[edit | edit source]
- ↑ a b c d e Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Sweizerische Gesellschaft für Ernährungforschung, Sweizerische Vereinigung für Ernährung. . Referenzwerte für die Nährstoffzufuhr (DACH). 1. vydání. Frankfurt am Main : Umschau/Braus, 2000. 216 s. ISBN 3-8295-7114-3
- ↑ a b ŠEBKOVÁ, Sylva. Otrava železem [online]. ©2003. Poslední revize 2003-10-06, [cit. 2021-08-16]. <http://medicina.cz/clanky/5819/34/Otrava-zelezem/>.
- ↑ a b c ANDERSSON, M, et al. Iodine Deficiency in Europe: A continuing public health problem [online] . 1. vydání. 2007. 70 s. Dostupné také z <http://www.who.int/nutrition/publications/micronutrients/iodine_deficiency/9789241593960/en/>. ISBN 978 92 4 159396 0.
- ↑ Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Iodine. SCF/CS/NUT/UPPLEV/26 Final 7 October 2002 http://ec.europa.eu/food/fs/sc/scf/out146_en.pdf
- ↑ World Health Organization. . Trace elements in human nutrition and health [online] . 1. vydání. Geneva : WHO, 1996. 160 s. Dostupné také z <http://apps.who.int/iris/bitstream/10665/37931/1/9241561734_eng.pdf>. ISBN 9241561734.
- ↑ (EDITOR), Richard D. Semba. Nutrition and Health in Developing Countries. 2. vydání. Totowa : Humana Press, 2008. 931 s. ISBN 978-1-934115-24-4.
- ↑ MASOPUST, Jaroslav a Richard PRŮŠA. Patobiochemie metabolických drah. 2. vydání. Univerzita Karlova, 2004. 208 s. s. 189−190.
- ↑ BENCKO, Vladimír, et al. Hygiena – učební texty k seminářům a praktickým cvičením. 2. vydání. Praha : Univerzita Karlova, 2002. 204 s. ISBN 80-7184-551-5.
- ↑ Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Sweizerische Gesellschaft für Ernährungforschung, Sweizerische Vereinigung für Ernährung. . Referenzwerte für die Nährstoffzufuhr (DACH). 1. vydání. Frankfurt am Main : Umschau/Braus, 2000. 216 s. ISBN 3-8295-7114-3.