Iron
Iron is one of the most important elements in the human body. The adult body contains more than 70 mmol (4.0-4.5 g) of iron. In women, this amount is lower than in men, which is attributed to blood loss during menstruation.
Distribution of iron in the body
| Form | Function | Protein | Amount in g |
|---|---|---|---|
| Active iron | oxygen transport | hemoglobin | 2.5-3.0 |
| myoglobin | 0.3 | ||
| electron transfer | cytochromes, cytochrome oxidase | 0.2 | |
| decomposition of hydrogen peroxide | catalase, peroxidase | ||
| Storage iron | ferritin, hemosiderin | 0.8–1.0 | |
| Transport iron | transferrin | 0.003 | |
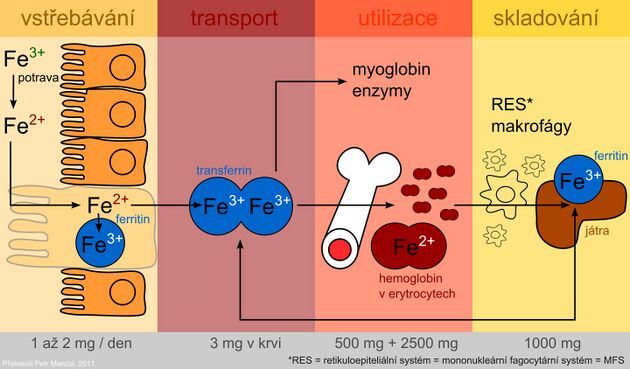
Metabolism of iron[edit | edit source]
Iron metabolism:
The presence of iron is essential for cell function. As a part of heme, it participates in the transport of oxygen and as a part of cytochromes it allows the transfer of electrons in the respiratory chain. However, the undesirable effect of iron is its involvement as a transient and highly reactive element of radical reactions, in which so-called reactive oxygen species (ROS) are formed. ROS can damage cell membranes, proteins and DNA.
Iron is absorbed in the Fe2+ form by active transport in the duodenum and upper jejunum by two mechanisms:
- Porphyrin-bound iron in the form of a stable lipophilic complex;
- Fe2+ water soluble chelates.
Only a small portion of the iron is absorbed in the ionized form.
The average diet provides approximately 10-50 mg of iron per day, but only 10-15% is absorbed. Iron in heme from meat products are absorbed by the human body better than non-heme iron from plant sources. In addition, plants contain oxalates, phytates, tannins and other phenolic compounds, which form insoluble or chelated complexes with iron that are difficult to be absorbed. On the other hand, ascorbic acid improves iron absorption.
- After uptake by the intestinal mucosa, part of the iron is incorporated into the storage form - with the protein ferritin in intestinal cells.
- Other portions of the absorbed iron passes into the plasma as well, where it is transported bound to transferrin.
- The protein ferroportin (it is also found in the membrane of macrophages and hepatocytes) plays an important role in the transfer of iron across the basolateral membrane of enterocytes. It is the main site of regulation of iron homeostasis in the body.
- A key regulatory factor is the hepcidin protein, which is synthesized in the liver. By binding to ferroportin, it inhibits the transport of iron from cells and thus contributes to its sequestration in them. Hepcidin levels increase with inflammation. Hepcidin is also partly responsible for anemia of chronic diseases. Mutations in the hepcidin gene lead to juvenile hemochromatosis type 2B .
Plasma iron is captured by target tissue cells via the transferrin receptor and is either incorporated into heme or stored in ferritin. The use of the specific transport protein transferrin and the ferritin storage protein for iron storage represents protective mechanisms to prevent the toxic effects of redox active iron.
Examination of iron metabolism[edit | edit source]
In medical practice, it is common to encounter diseases associated with changes in iron metabolism and utilization. Laboratory tests of iron metabolism include the following tests:
- Iron in serum
- Serum transferrin and iron binding capacity
- Serum ferritin
- Transferrin receptor
These parameters are important diagnostic indicators for demonstrating a decrease or increase in iron stores even in stages that are not accompanied by significant clinical manifestations.
Determination of iron in serum[edit | edit source]
Colorimetric methods, atomic absorption spectrophotometry and other special techniques are used to determine serum iron. The most used are photometric methods based on the reaction of iron with a complexing agent. All procedures include the following steps:
- Release of Fe3+ from transferrin binding using acids or surfactants (eg HCl).
- Reduction of Fe3+ to Fe2+, which is necessary for the reaction with the complexing agent. Ascorbic acid, for instance, is used for the reduction.
- Reaction of Fe2+ with a complexing agent containing reactive groups –N = C – C = N– is used to form a color complex. Metal ions form chelates with two nitrogen atoms. Currently, mainly two complexing agents are used - bathofenentroline and ferrozine (3- (2-pyridyl) -5,6-bis (4-sulfophenyl) -1,2,4-triazine - PST, protected name FerroZine®, which has higher absorption coefficient and is more soluble in water.
- Evaluation
- Serum iron concentrations are subject to circadian rhythm and are influenced by other factors. This limits the diagnostic significance of this parameter. It is a poor indicator of tissue iron stores and should always be considered in combination with serum transferrin and iron binding capacity.
- Decreased concentrations are accompanied by iron deficiency, caused for example, by large or repeated blood loss, insufficient dietary iron intake or impaired absorption. The finding is not specific, as reduced levels are also encountered in acute infection or chronic inflammatory diseases (iron transfer to tissues).
- High iron levels occur in hemochromatosis (see below), in iron overdose or intoxication, in increased erythrocyte breakdown, and in some liver diseases.
- Reference values
- Men: 9–29 μmol/L
- Women: 7–28 μmol/L
Serum transferrin and iron binding capacity[edit | edit source]
Iron is transported by the blood bound to a specific protein with β1-electrophoretic mobility, transferrin, which is synthesized in the liver.
- The rate of its formation is inversely proportional to the body's iron stores; it increases with iron deficiency and decreases with excess.
- The biological function of transferrin is the ability to easily form non-toxic iron complexes and transfer iron absorbed by the small intestinal mucosa to the bone marrow or storage forms (ferritin or hemosiderin).
- Each transferrin molecule binds two Fe3+ atoms (1 g transferrin binds 25.2 μmol iron).
- Transferrin can be determined directly by immunochemical methods or indirectly as the ability of transferrin to bind iron - the so-called iron binding capacity.
- Total iron binding capacity (TIBC ) is the amount of iron that transferrin is able to bind when all binding sites are occupied. Usually only 1/3 of transferrin-bound capacity is saturated with iron. Free transferrin without bound iron represents the free binding capacity (2/3 of transferrin) that is available for iron transport in increased demands.
Conversion between transferrin concentration and total binding capacity:
- Total binding capacity (μmol/L) = Transferrin (g/L) x 25.2
The reference range for serum transferrin concentration (S-transferrin) is 2.0–3.6 g/L and for a total binding capacity is 50–70 μmol/L.
Transferrin saturation[edit | edit source]
From the values of iron and transferrin concentration, we can calculate transferrin saturation (TfS), which is defined as the ratio of serum iron concentration to total iron transfer capacity for transferrin. This is a sensitive parameter for detecting latent iron deficiency.
- Evaluation of transferrin saturation
- Physiological values: 25-50%
- Iron deficiency saturation reduction: <15%
- Increase in saturation with excess iron: > 50%
Ferritin and hemosiderin[edit | edit source]
Ferritin is the most important storage protein for iron. The ferritin molecule is adapted to bind large amounts of Fe3+ in a soluble and non-toxic form to the body. It consists of an outer protein shell of 24 subunits - apoferritin (Mr 440,000), which up to 4500 iron atoms can be concentrated in the form of ferric oxyhydroxide (FeO · OH)n in the microcrystalline form with phosphates (FeO · OPO3H2). The entry and exit of iron atoms is enabled by the pores between the individual subunits of the ferritin shell. Normally, about 20% of its capacity is used. It is stored in the cells of the liver, spleen and intestinal mucosa.
Ferritin is found in very low concentrations in the blood serum. Serum ferritin concentrations are a measure of the body's iron stores. Low concentrations indicate depletion of the body's total iron reserve and serve to detect iron deficiency anemia early in the prelatent phase. Elevated ferritin concentrations are an accompanying phenomenon of high tissue iron stores. We also encounter them in many patients with liver disease, some malignancies (tumor marker) or inflammatory diseases (acute phase positive reactant).
The reference range for serum ferritin (S-ferritin) is 30-300 μg/L for men and 20-120 μg/L for women.
Hemosiderin is another form of storage of iron, derived primarily from the breakdown of erythrocytes. It is formed by aggregation of denatured ferritin with other components. It forms particles with a size of 1 to 2 μm, which are visible in a light microscope when iron staining is used. Hemosiderin contains more iron than ferritin, but is difficult to obtain due to its poor water solubility. It is formed when the amount of iron in the body exceeds the storage capacity of ferritin.
Transferrin receptor[edit | edit source]
Iron transported in blood by transferrin is taken up by cells via a specific transferrin receptor (TfR). It is on the surface of all cells at some stage of development, but is most expressed on the surface of erythrocyte precursors in the bone marrow. TfR is a transmembrane protein that is made up of two identical disulfide-linked subunits. By separating the extracellular domains of the receptor, the so-called soluble fraction of the transferrin receptor (sTfR), which may be in the form of a dimer or monomer, is released into the circulation. Cells respond to reduced iron stores by synthesizing increased levels of transferrin receptors.
An increase in sTfR could possibly indicate iron deficiency. Elevated sTfR levels are found in iron deficiency anemias or hemolytic anemias. Determination of sTfR levels can be used in patients with bone marrow transplantation to monitor the course of erythropoiesis.
The evaluation of of TfR levels utilise immunochemical methods.
Disorders of iron metabolism[edit | edit source]
Iron deficiency (sideropenia)[edit | edit source]
Iron deficiency in the body is usually caused by insufficient absorption from the intestine or chronic blood loss. It can result in sideropenic anemia (hypochromic microcytic anemia), which is one of the most common hematological diseases. However, anemia is usually a late symptom of progressive sideropenia. Therefore, it is necessary to detect iron deficiency at an early stage, when it is still not yet accompanied by anemia.
Iron deficiency can be classified into 3 types based on the laboratory results:
- Prelatant iron deficiency involves the decrease of storage iron, but the supply of iron to the bone marrow erythroblasts is still not affected. In about half of the patients, serum ferritin levels are reduced below 12 μg /L.
- Latent iron deficiency refers to the state where the iron reserves are essentially depleted. Ferritin is reduced below the lower normal limit, accompanied by a reduction in serum iron levels and a reduced supply to bone marrow erythroblasts. The binding capacity for iron increases. A sensitive indicator of latent iron deficiency is a decrease in transferrin saturation below 15%. However, anemia may not develop yet.
- Manifest iron deficiency anemia develops with hemoglobin levels falling below the lower limit of the normal range. Iron deficiency anemia is characterized by low serum iron and ferritin, and an increase in transferrin concentration (iron binding capacity). In contrast, in hemolytic anemias or in excess of iron, serum iron is increased, while the total iron binding capacity is reduced.
| Prelatant iron deficiency | Latent iron deficiency | Manifest iron deficiency |
|---|---|---|
| reduction of storage iron - decrease of ferritin | iron deficiency - decrease in ferritin | iron deficiency - decrease in ferritin |
| reduction of serum iron | reduction of serum iron | |
| transferrin saturation drop below 15% | transferrin drop below 10% | |
| increasing the overall binding capacity for iron | increasing the overall binding capacity for iron | |
| increase in sTfR | increase in sTfR | |
| reduction in hemoglobin - anemia |
Excess iron[edit | edit source]
The body is not equipped with an excretory pathway for iron, and therefore, under certain circumstances, excess iron may accumulate in the tissues. Early diagnosis can prevent tissue damage from excess iron. Iron overload usually develops very slowly. 3 stages could be distinguished:
- In the stage of prelatent iron surplus, iron content in the organs increases without exceeding their storage capacity.
- During the latent stage of iron overload, the storage capacity of the cells is exceeded, however, the function of the organs is still not yet impaired. The ferritin and serum iron levels increase and the transferrin saturation rises above 55%.
- In the phase of manifest iron surplus, some organs are already damaged.
| Prelatent excess of iron | Latent excess iron | Manifest surplus of iron |
|---|---|---|
| increasing iron reserves - increasing ferritin | increasing iron reserves - increase in ferritin above 300 μg/L | increase in iron reserves - increase in ferritin (in case of severe impairment above 2000 μg/L) |
| increase in serum iron | significant increase in serum iron | |
| increase in transferrin saturation above 55% | increase in transferrin saturation (may exceed 90% in severe disability) |
Hemochromatosis
The accumulation of iron in the tissues could be related to a disease known as hemochromatosis.
- Primary hemochromatosis is an inherited disease caused by increased resorption of iron in the gut. Excess iron is stored in parenchymal organs such as the liver, heart, pancreas and adrenal glands. The excess iron is toxic to the associated organs and disrupts their function by catalyzing reactions that lead to the formation of free radicals. The main clinical manifestations are skin hyperpigmentation, hepatosplenomegaly and diabetes mellitus.
- Secondary haemochromatosis may develop as a result of, for instance, repeated transfusions, excessive iron intake or haemolytic anemia. In the biochemical picture we find increasing levels of ferritin and iron in the serum, the saturation of transferrin increases with its simultaneous decrease.
Iron poisoning[edit | edit source]
Children are especially at risk of accidental ingestion of large quantities iron, which could lead to iron poisoning. This is not the issue of iron in regular diet, but rather, iron-containing supplements and multivitamins that could be mistaken as candies or other food by children.
The lethal dose of iron for a child is 600 mg. For adults, iron intake of 40 mg per kg of body weight is toxicologically severe, and an intake of 60 mg per kg of body weight is lethal.
Symptoms of iron poisoning include nausea, vomiting (including vomiting of blood), abdominal pain, diarrhea (could be bloody). Large fluid losses could cause shock, kidney failure and death. If the patient survives this phase of poisoning, he or she may become unconscious or convulsive after 12 hours. If he survives this second phase, the poisoning can have permanent consequences such as intestinal damage.
- Treatment of acute poisoning
- Gastric lavage.
- Usage of the nasogastral tube to deliver the chelating agent deferoxamine (5-10 g in 50-100 mL of water).
- Consider intravenous administration of desferoxanine to release absorbed iron. A pinkish red complex of deferoxamine with iron appears in the urine. Treatment should be repeated until urine returns to normal.
References and sources[edit | edit source]
- MASOPUST, Jaroslav a Richard PRŮŠA. Patobiochemie metabolických drah. 2. vydání. Univerzita Karlova, 2004. 208 s. s. 119–120.
- ŠEBKOVÁ, Sylva. Otrava železem [online]. ©2003. Poslední revize 2003-10-06, [cit. 2021-08-16]. <http://medicina.cz/clanky/5819/34/Otrava-zelezem/>.