Hyperbilirubinemia of newborns and infants
Hyperbilirubinemia is an increase in the level of bilirubin in the blood above 25 μmol/l. The level of conjugated (i.e. direct) but also unconjugated (i.e. indirect) bilirubin can increase in the blood. Unconjugated bilirubin is risky in terms of its ability to transfer through blood-brain barrier (BBB). In the brain, so-called nuclear jaundice can then occur. Bilirubin acts here on the basal ganglia, cranial nerve nuclei, cerebellum and auditory pathway.
It is also important to differentiate between physiological and pathological hyperbilirubinemia. We speak of pathological hyperbilirubinemia in the case of a specific increase in the level of conjugated and unconjugated bilirubin. Physiological hyperbilirubinemia is characterized by a transient increase in unconjugated bilirubin.
It is necessary to add that the physiological level of bilirubin is not harmful, it is part of the natural protection against oxygen radicals.
Icterus (jaundice) is a yellow discoloration of the sclera, later also of the skin and mucous membranes, which is usually visible at values above 68–85 µmol/l. Physiological hyperbilirubinemia (icterus neonati) affects 45-65% of healthy newborns.[1] The most common physiological cause is reduced ability to conjugate bilirubin with low glucuronyltransferase activity in the liver, shorter survival of erythrocytes and increased enterohepatic circulation of bilirubin.
The approximate value of bilirubin can be determined using "transcutaneous bilirubinometry" (TkB). With a simple look, for example, we can notice an "orange coloration" of the skin indicative of unconjugated hyperbilirubinemia or a "green-yellow coloration" indicative of conjugated hyperbilirubinemia. However, physical examination cannot distinguish these two conditions with certainty.
Bilirubin[edit | edit source]
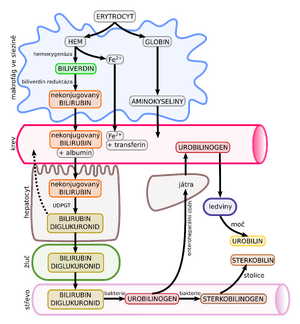
It is the final degradation product of hemoglobin. The main source is erythrocytes removed from the circulation and destroyed by the reticuloendothelial system. Unconjugated bilirubin enters the blood, which binds to albumin. The latter has a certain binding capacity that can be exceeded. Indirect bilirubin is fat soluble, hence the ability to settle in fatty tissues and the CNS. This bilirubin is then taken up in the liver, subsequently conjugated with glucuronic acid to form direct bilirubin. Conjugated bilirubin becomes water soluble by this process. It is then excreted into the bile.
Metabolism of bilirubin in the newborn[edit | edit source]
During the fetal period bilirubin is transported "transplacentally" because the fetal liver is not very active. After delivery, there is a rapid increase in bilirubin concentration (shorter erythrocyte life span, larger blood volume, minimal GIT passage and reabsorption of unconjugated bilirubin from the intestine). At the same time, the conjugation system of the liver is still ""immature".
Factors affecting bilirubin toxicity[edit | edit source]
Only the so-called free bilirubin - that part of unconjugated bilirubin that is not bound to albumin is toxic to the CNS. So one of the factors is the level of this bilirubin in the blood. It also depends on the albumin binding capacity which is reduced by acidosis, sepsis, immaturity and albumin binding drugs. These include furosemide, ampicillin or gentamicin. Free bilirubin is also more toxic when permeability of increases blood-brain barrier increases, which can lead to hypoxemia or hypercapnia as well as BBB immaturity itself. The last factor is then the individualsusceptibility of nervous tissue to damage by bilirubin.
Physiological hyperbilirubinemia[edit | edit source]
Physiological hyperbilirubinemia is defined as a transient increase in unconjugated (indirect) bilirubin. It does not require treatment.[1]
Criteria[edit | edit source]
This status must meet certain conditions. Starts after > 24 hours of age, the maximum bilirubin level is between 3. up to the 5th day of life and without intervention it starts to decrease within 10 days. Hyperbilirubinemia itself resolves within 14 days (except for jaundice from breast milk). The bilirubin values rise by less than 85 μmol/l/day and are generally at least 50 μmol/l below the limit of phototherapy, which we can verify with the indicator graph. Less than 20% of total bilirubin is conjugated with values < 34 μmol/l. We do not find signs of hemolysis, biliary tract obstruction or other pathological symptomsin the newborn. These include dehydration/hydrops, bleeding, pallor, plethora, hepatosplenomegaly, splenomegaly, sepsis, positive TORCH, slowed peristalsis or bilirubin encephalopathy. According to Hodros chart, this is at most icterus II. bands.
For premature newborns, the same criteria apply with slight variations. The maximum level of bilirubin only occurs between 5. and day 7 of life, decline without intervention appears within 14 days and the condition resolves within 21 days.
Causes[edit | edit source]
The reason for the development of this condition can be a reduced elimination capacity of bilirubin in the liver (especially reduced uridine diphosphoglucuronyltransferase), a higher breakdown of erythrocytes after birth, a higher percentage of bilirubin not originating from erythrocytes, an increased activity of beta-glucuronidase in the intestine orhigher enterohepatic circulation of bilirubin.
Jaundice of a breastfed baby[edit | edit source]
A specific subcategory of physiological hyperbilirubinemia is jaundice of a breast-fed infant. It occurs in healthy, breast-fed full-term infants who are thriving, have normal liver function tests, and do not have elevated conjugated bilirubin. We meet the maximum level of bilirubin on the 5th to 6th day of life (sometimes up to the end of the 2nd week). Bilirubin decreases more slowly and 25-50% passes into prolonged jaundice. Jaundice resolves spontaneously within 1-4 months.
Summary[edit | edit source]
| Mature baby | Premature baby | Breastfed | |
|---|---|---|---|
| Maximum level | Day 3-5 | Day 5-7 | Day 5-6 (end of week 2) |
| Decrease in bilirubin | within 10 days | within 14 days | slower than the previous |
| Retreat | within 14 days | within 21 days | 1-4 months |
Pathological hyperbilirubinemia[edit | edit source]
Pathological hyperbilirubinemia can manifest itself in various symptoms. These include, for example, jaundice in the first 24 hours of life or jaundice in a sick newborn. In the laboratory, we can observe an increase in total bilirubin to > 250 µmol/l within 48 hours of life or > 300 µmol/l within 72 hours of life, bilirubin also increases rapidly, by > 100 µmol/l/24 h. Jaundice often does not respond to phototherapy and may be prolonged (> 14 days in full-term and > 21 days in premature neonates). The level of conjugated bilirubin can be found above 25 µmol/l. Other signs can be light to acholic stools and dark urine (in a child not on phototherapy).[2]
We distinguish 2 states:
- Persistent icterus lasts more than 1-2 weeks in a full-term infant, 2-3 weeks in a premature infant (it can easily persist for 2-3 months in fully breastfed).
- Prolonged icterus peaks in the 1st week of life and does not decrease even in the 2nd and 3rd weeks of life (in fully breastfed newborns it can persist for 4 months).
Causes of unconjugated hyperbilirubinemia of the newborn[edit | edit source]
Unconjugated hyperbilirubinemia can arise on various grounds. The first one is hemolysis. Furthermore, polycythemia, extravasation of blood, increased enterohepatic circulation may be involved., Crigler-Najjar syndrome or endocrine/metabolic disorder. See the table for the causes of individual conditions.
| Examples | |
|---|---|
| Hemolysis | isoimmunization (Rhesus, AB0, minority groups such as Kell), spherocytosis, glucose-6-phosphate dehydrogenase deficiency, pyruvate kinase deficiency, sepsis, disseminated intravascular coagulopathy, alpha-thalassemia |
| Polycythemia | hypotrophy, twin transfusion syndrome, delayed umbilical cord interruption, maternal-fetal transfusion, newborn of a diabetic mother |
| Extravasation of blood | hematomas, cephalhematoma, pulmonary hemorrhage, intracranial hemorrhage, intra-abdominal bleeding |
| Increased enterohepatic circulation | pylorostenosis, intestinal obstruction, swallowed blood |
| Endocrine/metabolic disorder | hypothyroidism, hypopituitarism, hypoadrenalism, glucuronosyltransferase deficiency, galactosemia (hepatomegaly, feeding problems, vomiting), tyrosinemia, hypermethioninemia |
note: DGlucose-6-phosphate dehydrogenase efficiency is mainly found in newborns of the male sex from the Mediterranean, Asia and Africa. Manifestation usually occurs on the 3rd-5th day of life.
In Crigler-Najjar syndromenon-hemolytic unconjugated hyperbilirubinemia rapidly develops in the first days of life. We recognize 2 types of this syndrome. Type I is characterized by the absence of UDPGT in hepatocytes, is inherited by the AR, and requires repeated exchange transfusion and liver transplantation. Type II only has reduced activity of UDPGT and serum bilirubin can be reduced by phenobarbital which induces UDPGT.[2]
Causes of conjugated hyperbilirubinemia of the newborn[edit | edit source]
Conjugated hyperbilirubinemia has many causes. These include long-term parenteral nutrition or cholestasis, namely idiopathic neonatal, or progressive familial intrahepatic. Other diseases leading to this condition are perinatal asphyxia, severe hemolysis, various types of infection, obstruction of the bile ducts, spontaneous perforation of the bile duct, intrahepatic biliary hypoplasia (so-called Alagill syndrome), alpha1-antitrypsin deficiency, cystic fibrosis , galactosemia and tyrosinemia.[2]
There are many pathogens involved in the infection, for example bacterial sepsis or intrauterine infection, known by the abbreviation CHEAP TORCHES (an extended version of TORCH).
Bile duct obstruction arises, for example, on the basis of biliary atresia, which is manifested by acholic stools and is corrected surgically. Another reason can be biliary plug syndrome or choledochus cyst. It is characterized by the dilation of the bile ducts, which intermittently cause biliary obstruction. As with biliary atresia, it is treated surgically.
Spontaneous perforation of the bile duct, which manifests itself as mild icterus, ascites and failure to thrive, is also treated surgically.
Summary[edit | edit source]
Differentiating diagnostics is broad, but we can use the aid HADI S MECHEM C.
H = hepatitis (congenital infection idea, see cheap torches)
A = biliary atresia
D = drugs (ceftriaxon, which creates "sludge" in the gallbladder, problems disappear after stopping)
I = idiopathic
S = sepsis
M = metabolic causes (eg galactosemia, Niemann-Pick, Gaucher, and others)
E = endocrine causes (hypothyroidism, panhypopituitarism)
CH = chromosomal malformation (Down et Edwards disease)
E = extrahepatic pathology (biliary obstruction, neonatal sclerosing cholangitis)
M = biliary tract malformation (hypoplasia, choledochal cyst, pancreaticobiliary tree anomaly)
C = cystic fibrosis
Kernicterus[edit | edit source]
Kernicterus is a very serious but rare complication of hyperbilirubinemia. It is formed by the deposition of unconjugated bilirubin in the brain tissue, basal ganglia and brainstem. The exact level of bilirubin that leads to kernicterus is not known. Bilirubin toxicity is influenced by a number of factors - gestational age, ethnicity, presence of hemolysis, asphyxia, acidosis, hypoperfusion, hyperosmolality, sepsis. Mild, non-specific symptoms of bilirubins toxic effects on the central nervous system first appear: lethargy, feeding problems, high pitched crying and hypotonia. After about a week, increased irritability, opisthotonus, convulsions, apnea, hypertension, temperatures develop. Subsequently, there is the development of chronic encephalopathy, in the form of cerebral palsy (athetoid form), mental retardation, dental dysplasia, hearing impairment up to deafness and paralysis of the oculomotor muscles.[1]
Examination of an icteric newborn[edit | edit source]
In the examination, we focus on family and obstetric anamnesis. We also carry out clinical and laboratory tests. The time of manifestation of the first clinical manifestations is also important.
Anamnesis[edit | edit source]
We are looking for jaundice, anemia and splenectomy in the family. We detect hepatitis and syphilis in the mother.
In the birth anamnesis, we focus on the mechanism of delivery, occurrence of cephalhematoma and asphyxia. We are also interested in whether the newborn was full-term (prematurity) or whether the mother has diabetes mellitus.
Physical examination[edit | edit source]
During the physical examination, the child may "appear healthy" (malformation of the biliary tract, drugs, idiopathic cause), or, on the contrary, show "signs of alteration" (hepatitis within TORCH, sepsis, metabolic defects, chromosomal aberration ). Babies with biliary atresia look physiological after birth.
We often find hepatomegaly or hepatosplenomegaly. Specific is dark colored urine and hypocholic to acholic stools (light, yellow-white). Next, we focus on the presence of jaundice, evaluate pallor, look for tachycardia, thermability or dyspnea. A newborn may also have problems with food (loss of appetite, apathy when drinking).
Non-invasive "transcutaneous icterometry" is used for indicative examination of the bilirubin level.
Laboratory examination[edit | edit source]
- total and conjugated bilirubin, blood type and Rh factor, direct Coombs test (detects antibodies against erythrocytes);
- blood count incl. reticulocytess (> 6% after the 3rd day of life is indicative of hemolysis)[2] and differential, erythrocyte morphology (slide smear), hemoglobinopathy, enzymopathy;
- CRP, liver enzymes, ABR, glycemia, TORCH serology (CMV in urine), examination of metabolic defects;
- cholesterol, total protein, albumin, hemocoagulation test, lactate, ammonia, fT4, TSH, α1</ sub>-antitrypsin;
- urine chemically and urinary sediment;
- urine culture, swabs, blood culture.
Laboratory examination of prolonged hyperbilirubinemia[edit | edit source]
- Blood count, blood group, Rh factor and direct Coombs test, total and conjugated bilirubin, fT4, TSH, (or liver tests, α1-antitrypsin, cystic fibrosis screening (part of newborn laboratory screening from a dry drop), cortisol, immunoreactive trypsin, examination of serum amino acids);
- urine − culture, proof of reducing substances (galactosemia).[2]
Targeted examination[edit | edit source]
If the cause of jaundice is suspected, we will perform targeted sampling.
| Cause | Examination |
|---|---|
| Evidence of cholestasis and liver function | conjug. and total bilirubin, ALT, AST, GMT, cholinesterase, albumin, ammonia, INR |
| Sepsis | zz.parameters, HK, ev. other cultures (urine, CSF) |
| Neonatal hepatitis | serology |
| Metabolic defects | metabolic screening |
| Cystic fibrosis | chlorides in sweat |
| Hypothyroidism | fT4 a TSH |
| Panhypopituitarism | kortisol, IGF-1, FSH, LH |
| Chromosomal aberrations and monogenic causes | karyotype and molecular genetic examination |
To evaluate the overall condition we use the detection of ions, renal functions and glycemia.
Imaging methods[edit | edit source]
Imaging methods are used firstly for the diagnosis of associated defects, then we perform an ultrasound of the liver, an X-ray of the chest and echocardiography (e.g. Alagille syndrome = cholestasis + butterfly-shaped vertebrae + heart defect + triangular face), on the one hand, to evaluate the state of the bile ducts (atresia and other malformations), for this purpose, the ERCP is used.
Liver biopsy[edit | edit source]
Liver biopsy is indicated in case of bile duct atresia, hepatitis grading and neonatal sclerosing cholangitis.
Therapy of unconjugated hyperbilirubinemia of the newborn[edit | edit source]
We treat the underlying disease. The goal is to prevent bilirubin levels that would put newborns at risk of developing nuclear jaundice. Bilirubin can be eliminated using phototherapy, exchange transfusion, or pharmacotherapy.
Therapy is guided by total bilirubin levels. The method of therapy is determined by indicative charts which take into account the gestational age of the newborn, postnatal age (number of hours since birth) and the presence of risk factors. There are a number of charts that differ slightly from each other. In the Czech Republic, the Hodr-Poláček indicator chart or the recommendations of the American Pediatric Association (APA) are often used.[1]
Phototherapy[edit | edit source]
It is recommended to start phototherapy at values above 350 µmol/l at the age of 72 hours, (at a younger age earlier). For the phototherapy treatment of 96 hours old newborns, these values are above 320 µmol/l (for premature babies above 260 µmol/l). The most effective is blue light, whose wavelength is closest to the absorption spectrum of bilirubin (460 nm). During phototherapy certain precautions are necessary: covering the eyes, monitoring vital functions, ensuring normothermia, sufficient hydration and nutrition.
Exchange transfusion[edit | edit source]
Exchange transfusion can be considered at a level above 450 µmol/l. We remove sensitized erythrocytes, antibodies, bilirubin and toxic substances. After the procedure, it is necessary to correct the anemia. In case of AB0 incompatibility, erythrocytes 0 in AB plasma are administered. In case of Rh incompatibility, the childs blood type is given, Rh negative. A screening examination of hearing and monitoring of psychomotor development is recommended.
Pharmacotherapy[edit | edit source]
- Isoimmune hemolytic disease − intravenously administered "immunoglobulins" bind circulating maternal antibodies and thus reduce the risk of hemolysis.
- Crigler-Najjar syndrome type II − administration of phenobarbital to induce UDP-glucuronosyltransferase.[1]
Links[edit | edit source]
Related articles[edit | edit source]
- Jaundice (pediatrics) ▪ Hemolytic disease of the newborn ▪ Juvenile hyperbilirubinemia
- Icterus ▪ Differential diagnosis of jaundice ▪ Neonatal cholestasis
- Blood groups • Inheritance of blood groups • Rh system • AB0 system
External links[edit | edit source]
Recommended practices[edit | edit source]
- ČNeoS: Doporučené postupy v neonatologii - Hyperbilirubinemie novorozence
- AAP: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation (2004)
- AAP: Hyperbilirubinemia in the Newborn Infant ≥35 Weeks’ Gestation: An Update With Clarifications (2009)
- NICE: Neonatal jaundice (2010)
Articles[edit | edit source]
Reference[edit | edit source]
Source[edit | edit source]
- BENEŠ, Jiří. Studijní materiály [online]. ©2007. [cit. 2009]. <http://www.jirben.wz.cz/>.
Literature[edit | edit source]
- HRODEK, O – VAVŘINEC, J. Pediatrie. - edition. Galén, 2002. 767 pp. ISBN 80-7262-178-5.
- ŠAŠINKA, M. Pediatria, zv. I a II. - edition. Satus, 1998. ISBN 80-967963-0-5.