Hemolytic disease of the newborn
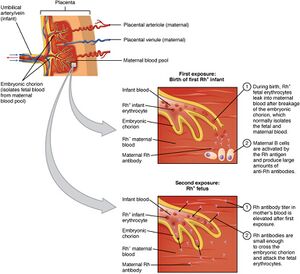
Hemolytic disease of the fetus and newborn (HDN) or fetal erythroblastosis (ICD-10) is an intrauterine damage fetus with maternal antibodies. It is the most common cause of pathological unconjugated hyperbilirubinemia. It is most often caused by Rh system incompatibility. This is a situation where an Rh-negative mother has an Rh-positive child with an Rh-positive partner. Under normal circumstances, the blood of the mother and the fetus do not mix. Penetration of fetal blood into the mother's circulation occurs during childbirth, abortion, ectopic pregnancy, premature separation of the placenta, amniocentesis and other situations. As soon as the mother's organism meets the Rh-positive erythrocytes of the fetus (i.e. most often during childbirth), it creates so-called anti-D-antibodies, which then easily penetrate the placenta into the circulation during the next pregnancy the fetus, they bind to the erythrocytes of the fetus and cause their accelerated uptake and destruction in the spleen (hemolysis), which manifests itself as newborn jaundice. The fetus is already at risk during the second half of pregnancy. With a severe course of Rh-incompatibility, hydrops congenitus universalis can occur. Incompatibility in the AB0 system can manifest itself already in the first pregnancy, but the fetus is usually not at risk [1][2][3].
Thanks to the routine examination of the blood group of pregnant women and the prophylactic administration of anti-D immunoglobulins to Rh-negative women, the incidence of hemolytic disease of the newborn due to Rh isoimmunization has decreased. Other causes of HNN include anti-c, Kell (K and k), Duffy (Fya), Kidd (Jka and Jkb), MNS (M, N, S and s), anti-C and anti-E[4]. Fetal erythroblastosis Brief characteristics:
- positive Coombs test (proof of antibodies);
- hyperbilirubinemia;
- anemia.
Rh incompatibility[edit | edit source]
Hemolytic disease of the newborn in the Rh system is most often caused by anti-D antibodies that pass through the placenta, bind to fetal erythrocytes and cause their premature destruction. Erythropoiesis increases in the bone marrow and extramedullary, but it is not enough to replace the broken erythrocytes, so anemia develops with variously expressed consequences:
- unconjugated hyperbilirubinemia in the first 24 hours of life
- the bilirubin level is already high in the umbilical cord blood and rises rapidly,
- icterus appears already in the first hours after birth,
- without adequate treatment, there is a risk of developing kernicterus,
- hemoglobin is in the normal range or reduced.
- severe anemia
- the child is noticeably pale after birth, hemoglobin is 50-100 g/l,
- increased extramedullary hematopoiesis in the liver, spleen (hepatosplenomegaly), skin, etc.
- hydrops - the most severe form of HNN
- extreme anemia causes chronic tissue hypoxia, hypoproteinemia, heart failure and generalized swelling with accumulation of fluid in body cavities,
- can cause the fetus to die[2].
For alloimmunization, it is sufficient for 0.1 ml of Rh-positive erythrocytes (fetus) to pass into the Rh-negative circulation (mother). The mother's organism starts to produce first IgM, which do not pass through the placenta, and then begins to produce IgG, which passes through the placenta. Some Rh-negative mothers do not develop specific anti-Rh IgG despite repeated exposure to Rh antigens[5][4].
Concomitant AB0 incompatibility partially reduces the risk of maternal Rh sensitization because fetal erythrocytes are destroyed by antibodies in the AB0 system after passing into the maternal circulation before an immune response to the presence of the Rh antigen is triggered[5].
Diagnosis[edit | edit source]
- examination of the blood group of the mother – it is part of prenatal care, in case of Rh negativity, anti-D antibodies are also examined;
- in risk newborns, umbilical cord blood examination: child's blood group, Coombs test, bilirubin, hemoglobin;
- direct Coombs test or direct antiglobulin test (proof of antibodies bound to the baby's erythrocytes);
- after administration of antenatal anti-D prophylaxis, these anti-D IgGs may enter the fetal circulation and cause a false-positive Coombs test result, however, these prophylactic IgGs do not cause hemolysis of fetal erythrocytes[4];
- direct Coombs test or direct antiglobulin test (proof of antibodies bound to the baby's erythrocytes);
- further monitoring of the level of unconjugated bilirubin and anemia (including the number of reticulocytes)[3][2].
Treatment[edit | edit source]
- phototherapy or exchange transfusion (according to the level of unconjugated bilirubin - it follows the indication chart, in the Czech Republic usually according to Poláček and Hodra);
- intravenous immunoglobulins – can slow down the mass breakdown of sensitized erythrocytes
- hydrops universalis – umbilical vein catheterization, ascites puncture, transfusion of 0-Rh-negative erythrocytes and exchange transfusion
Prevention[edit | edit source]
- Anti-D-prophylaxis: administration of anti-D to Rh-negative mothers in the 28th week of pregnancy and within 72 hours after Rh-neg. the mother gives birth Rh-positive. baby, the mother receives immunoglobulins with a high content of anti-Rh antibodies to destroy the Rh-positivity. erythrocytes in her circulation before the mother has time to develop antibodies against them (anti-D).[2]</ref>[3][6]
- However, the mother's Rh immunization does not have to happen until after the first birth, but in any situation where Rh+ erythrocytes have penetrated the bloodstream of the Rh- mother, against which the mother has subsequently developed antibodies (anti-D Ig). This can occur after an Rh+ blood transfusion or after an abortion of an Rh+ fetus. The information that this is the first pregnancy is extremely unreliable in terms of the risk of fetal erythroblastosis. Therefore, all Rh- pregnant women should be repeatedly tested for the presence of anti-D antibodies.
AB0 incompatibility[edit | edit source]
- AB0 incompatibility is present in 20-25% of pregnancies, but symptoms appear in only 10% of cases[3];
- the mother has IgM-isoantibodies against A and B even without immunization, but these antibodies cannot cross the placenta (IgM antibodies are pentamers, IgG are monomers);
- if the mother develops IgG antibodies, they can already pass through the placenta;
- hemolysis is less pronounced than in Rh-incompatibility, because part of the anti-A and anti-B antibodies is neutralized by AB-antigens in the placenta and not all fetal erythrocytes express A- or B-antigens.[3]
Clinical picture[edit | edit source]
- mild anemia, rarely hepatosplenomegaly;
- hyperbilirubinemia with the risk of nuclear icterus (kernicterus).
Treatment[edit | edit source]
- According to the level of unconjugated bilirubin - no. phototherapy.
References
Related Articles[edit | edit source]
- Rh system • Blood groups • Inheritance of blood groups
- Hyperbilirubinemia of neonates and infants • Exchange transfusion
External links[edit | edit source]
References[edit | edit source]
- ↑ http://www.porodnice.cz/poradny/dobry-den-jsem-v-28tt-absolvovala
- ↑ a b c d yes. . Neonatology : selected chapters for students of LF. 1. edition. Prague : Karolinum, 2005. ISBN 80-246-0790-5.
- ↑ a b c d e ano. . Pediatrics. 4. edition. Prague : Grada, 2009. pp. 21-23. ISBN 978-80-247-2525-3.
- ↑ a b c ano. . Neonatology : Management, Procedures, On-Call Problems, Diseases, and Drugs. 6. edition. Lange, 2009. pp. 654-658. ISBN 978-0-07-154431-3.
- ↑ a b ano. . Neonatology : Management, Procedures, On-Call Problems, Diseases, and Drugs. 6. edition. Lange, 2009. pp. 500. ISBN 978-0-07-154431-3.
- ↑ JANOTA, Jan – STRAŇÁK, Zbyněk. Neonatology. 1. edition. Prague : Mladá fronta, 2013. pp. 401. ISBN 978-80-204-2994-0.