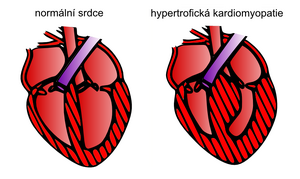
Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy is one of the most common types of Cardiomyopathy with a frequency of 1:500 [1][2] and also one of the most frequent causes of sudden death in young individuals and athletes. It is characterized by thickening (hypertrophy) of the myocardium without the presence of left ventricular dilatation, while other pathologies that could explain the myocardial hypertrophy are excluded (e.g. arterial hypertension, aortic valve stenosis, ischemic heart disease etc.).[1]
The most common cause of hypertrophic cardiomyopathy is mutation of genes encoding the sarcomeric components of cardiomyocytes.[1] However, the situation is complicated by the fact that a specific mutation is only identified in approximately 50% of patients and also by the occurrence of mutations/variations of unclear significance.[1]. In some cases, hypertrophic cardiomyopathy also occurs in metabolic and neuromuscular disorders (Fabry's disease, Friedreich's ataxia).[1][2]
The clinical picture is diverse, including asymptomatic cases and patients in whom the first manifestation is malignant arrhythmia or sudden cardiac death, which is also one of the main dangers of this disease. The trigger can be extreme physical exertion or a sudden change in ion level. Hypertrophic cardiomyopathy is accompanied by diastolic dysfunction (poor relaxation of the hypertrophic myocardium) and, in the vast majority of cases, hyperdynamic function of the left ventricle with its intact systolic function. Due to the genetic basis of the disease, whose inheritance is autosomal dominant with high penetrance, other family members are also examined as part of the cascade screening. [1][2]
Etiopathogenesis[edit | edit source]
Hypertrophic cardiomyopathy is mostly a genetically determined disease with an autosomal dominant type of inheritance and high penetrance, and therefore a frequent familial occurrence. Several hundred genes associated with this cardiomyopathy are known.[3] Mutations most frequently affect the genes for contractile [[Skeletal muscle structure|myofilaments] of the sarcolemma of cardiomyocytes (beta myosin heavy chain and myosin-binding protein C).[1]
In 5-10 %[3] of adult patients, the etiology is a sparing, mitochondrial or neuromuscular disorder such as Fabry's disease, Friedreich's ataxia, Danon's disease, etc. In children, the representation of these pathologies in the etiology of hypertrophic cardiomyopathy is up to 25%.[3] In general, the diagnosis of these forms is based on genetic, laboratory or biopsy examination of patients. Treatment is mostly supportive and symptomatic.[4] [5]
Histopathological and functional changes of the myocardium[edit | edit source]
Macroscopically myocardial hypertrophy, is present , which can also be concentric. It most often affects the interventricular septum and the wall of the left ventricule.[1] Pathology of the suspension apparatus of the mitral valve related to the development of obstruction of the outflow tract of the left ventricle is very common. This happens due to the lengthening of both or one of the mitral valve leaflets, which leads to an overall enlargement of the valve.[1]
Microscopically, disorganization of muscle bundles, hypertrophy of cardiomyocytes and interstitial fibrosis are visible. Thickening of the walls of intramural coronary arterioles and narrowing of the vessel lumen are also identifiable, which is probably responsible for repeated clinically silent myocardial ischemia leading to the mentioned interstitial fibrosis.[1]
A very common form of hypertrophic cardiomyopathy is the so-called hypertrophic cardiomyopathy with obstruction of the outflow tract of the left ventricle, when the contraction of the hypertrophic septum and the abnormal movement of the front leaflet of the mitral valve towards the septum (systolic forward movement) cause partial obstruction of the outflow tract of the left ventricle during systole.[5] Obstruction, both at rest and upon provocation, is detectable in approximately 70% of cases and plays a significant role in the development of heart failure and patient prognosis.[1]
Left ventricular diastolic dysfunction, is also usually present, which in some patients is the basis for the development of heart failure with a preserved left ventricular ejection fraction.[1] Left ventricular systolic failure may also develop in a small percentage of cases.[1][3]
Epidemiology[edit | edit source]
Hypertrophic cardiomyopathy is one of the most common genetically determined cardiovascular diseases with an incidence of 1:500.[1][4] In 5-10% of adults, or In 25% of children, however, its etiological basis is various accumulative or neurodegenerative diseases.[3][5] Fabry disease with an epidemiology of 1:40,000 - 1:117,000 in men accounts for 1% of the overall etiology of hypertrophic cardiomyopathy.[4]
Clinical picture[edit | edit source]
Hypertrophic cardiomyopathy can manifest at any time during an individual's life.[1][2][6] Patients complain of chest pain and exertional dyspnea (even one of Angina pectoris character), palpitations, ortopnea, syncopal or presyncopal states.[1][2][5][6]
Some patients develop obstruction of the outflow tract of the left ventricle. This condition can occur already during rest, or during provocative maneuvers. Obstruction can worsen or manifest when standing, with tachycardia, hypovolemia, Valsalva maneuver, nitrates, after positive ionotropic substances (digoxin).[1][2]
Physical examination findings are variable and do not include any specific sign. In most patients with left ventricular outflow tract obstruction, an ejection systolic murmur is present over the apex and at the auscultatory site of the aortic valve.[1][2] This finding is similar to that of aortic stenosis, however, in the case of hypertrophic cardiomyopathy, murmur radiation is absent and the murmur also shows considerable variability in intensity depending on the subaortic gradient.[1]
Patient follow-up and sudden death risk stratification[edit | edit source]
The majority of patients have a good prognosis, without the development of serious pathologies. In some cases, they have relatively mild symptoms of the disease, which can often be influenced by appropriately chosen therapy and other interventions.
![]() První manifestací HKMP ale může být i náhlé úmrtí nebo vznik maligních arytmií.
První manifestací HKMP ale může být i náhlé úmrtí nebo vznik maligních arytmií.
It is the most common cause of sudden death in athletes and young individuals[1][2][6] (e.g. non-sustained ventricular tachycardia detected in up to 20-30% of cases[6]).
The principle of HKMP therapy is to stratify according to possible risk and then provide therapeutic care for patients with an increased risk of sudden death, development or progression of heart failure, or the occurrence of atrial fibrillation (up to 25%).[1][6]
Diagnostics[edit | edit source]
The diagnostic process includes both a basic ECG and echocardiographic examination, as well as other imaging techniques such as magnetic resonance of the heart, CT or Coronary angiography. Genetic testing, also plays an important role , which is also important for the cascade screening of the patient's relatives.
ECG, imaging and laboratory methods[edit | edit source]
Pathological changes are visible on the ECG which may resemble a myocardial Infarction. The voltage criteria of left ventricular hypertrophy, up to deep inversion of the T wave (and other ST-T section changes) and Q wave are often present . Regular Holter monitoring is also important to detect malignant arrhythmias.
Echocardiographic examination is primary, where the most common finding is asymmetric hypertrophy of the septum, the thickness of which in some cases reaches 20 mm or more. A thickening of more than 15 mm is diagnostic for hypertrophic cardiomyopathy, but suspicion sometimes arises as early as 13 mm.[1] In connection with the mitral valve, or even by obstruction of the outflow tract of the left ventricle, the systolic forward movement of the anterior leaflet of the mitral valve abbreviated as SAM) is described . The heart may not be enlarged on an X-RAY.
In monitoring patients he occurrence of arrhythmias and the dynamics of changes in myocardial hypertrophy and obstruction of the outflow tract of the left ventricle are monitored. Laboratory monitoring of cardiospecific enzymes and natriuretic peptides is also common.[3]
Therapy[edit | edit source]
The primary goal of therapy is to prevent Sudden cardiac death and treat symptoms. It consists of regimen measures, pharmacological treatment, anticoagulant and interventional therapy for atrial fibrillation ICD implantation when the indication criteria are met, and in selected patients from alcohol ablation of the septum or septal myectomy.[1][2][5][6]
Regime measures and pharmacological treatment[edit | edit source]
In general extreme physical activity and competitive sports should be excluded. It is also recommended to follow the principles of a healthy lifestyle and to abstain from smoking and to avoid alcohol or drug excesses, which could affect the balance of electrolytes and potentially cause malignant arrhythmia. Physically demanding work is also not recommended due to the risk of arrhythmias.[3]
Drug therapy may not be necessary in asymptomatic patients.[3][6] In order to influence the symptoms, or even prognoses beta-blockers, bcalcium channel blockers (verapamil), sometimes antiarrhythmics (disopyramid), diuretics (caution due to possible hypotension, hypovolemia and provocation of obstruction) and ACE inhibitors are used . Peripheral vasodilators are not recommended.[7] In patients with atrial fibrillation, anticoagulant treatment or cardioversion is indicated.[3]
Treatment and prevention of arrhythmias and sudden death[edit | edit source]
Medical antiarrhythmic treatment consists of therapy with beta-blockers or amiodaron. In higher-risk patients, the indication for an implantable cardioverter-defibrillator (ICD) v rámci primární nebo sekundární prevence (setrvalé komorové tachykardie, srdeční zástava apod.) is considered as part of primary or secondary prevention (sustained ventricular tachycardia, cardiac arrest, etc.). There are many factors that play a role in the indication.[1][2]
Surgical and invasive treatment[edit | edit source]
The surgical solution involves septal myectomy, which is often combined with mitral valve replacement. An invasive solution is percutaneous transluminal septal myocardial ablation (PTMSA). Patients experience symptomatic relief as a result of the reduction in the gradient of obstruction, and a reduction in the risk of sudden death has also been described.[5]
Links[edit | edit source]
Related articles[edit | edit source]
Sarcomeric and non-sarcomeric forms of hypertrophic cardiomyopathy
Used literature[edit | edit source]
- MANN, Douglas L., et al. Braunwald´s Heart Disease : A Textbook of Cardiovascular Medicine. 10. edition. 2015. ISBN 978-0-323-29429-4.
- STANĚK, Vladimír. Kardiologie v praxi. 1. edition. Axonite CZ, 2014. 375 pp. ISBN 9788090489974.
- KUUSISTO, Johanna – SIPOLA, Petri – JÄÄSKELÄINEN, Pertti. Current perspectives in hypertrophic cardiomyopathy with the focus on patients in the Finnish population: a review. Annals of Medicine. 2016, y. 7, vol. 48, p. 496-508, ISSN 0785-3890. DOI: 10.1080/07853890.2016.1187764.
- PALEČEK, T – KUCHYNKA, P. , et al. Nesarkomerické formy hypertrofické kardiomyopatie v dospělosti. Kardiologická revue - interní medicína. 2011, y. 13, vol. 4, p. 210-220, ISSN 2336-2898.
- VESELKA, Josef – ANAVEKAR, Nandan S. – CHARRON, Philippe. Hypertrophic obstructive cardiomyopathy. The Lancet. 2017, vol. 389, p. 1253-1267, ISSN 0140-6736. DOI: 10.1016/s0140-6736(16)31321-6.
- MARIAN, Ali J. – BRAUNWALD, Eugene. Hypertrophic Cardiomyopathy. Circulation Research. 2017, y. 7, vol. 121, p. 749-770, ISSN 0009-7330. DOI: 10.1161/circresaha.117.311059.
- PROF. MUDR. ŠTEJFA, Miloš, et al. Kardiologie. 3. edition. 2007. 776 pp. ISBN 978-80-247-1385-4.
- KAUTZNER, Josef. Srdeční selhání : aktuality pro klinickou praxi. 1. edition. Mladá fronta, 2015. ISBN 9788020435736.
- KUCHYNKA, P. Kardiomyopatie [online]. [cit. 2023-03-27]. <http://int2.lf1.cuni.cz/file/5727/kardiomyopatie-pro-mediky.pdf>.
External links[edit | edit source]
References[edit | edit source]
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x MANN, Douglas L., et al. Braunwald´s Heart Disease : A Textbook of Cardiovascular Medicine. 10. edition. 2015. ISBN 978-0-323-29429-4.
- ↑ a b c d e f g h i j STANĚK, Vladimír. Kardiologie v praxi. 1. edition. Axonite CZ, 2014. 375 pp. ISBN 9788090489974.
- ↑ a b c d e f g h i KUUSISTO, Johanna – SIPOLA, Petri – JÄÄSKELÄINEN, Pertti. Current perspectives in hypertrophic cardiomyopathy with the focus on patients in the Finnish population: a review. Annals of Medicine. 2016, y. 7, vol. 48, p. 496-508, ISSN 0785-3890. DOI: 10.1080/07853890.2016.1187764.
- ↑ a b c PALEČEK, T – KUCHYNKA, P. , et al. Nesarkomerické formy hypertrofické kardiomyopatie v dospělosti. Kardiologická revue - interní medicína. 2011, y. 13, vol. 4, p. 210-220, ISSN 2336-2898.
- ↑ a b c d e f VESELKA, Josef – ANAVEKAR, Nandan S. – CHARRON, Philippe. Hypertrophic obstructive cardiomyopathy. The Lancet. 2017, vol. 389, p. 1253-1267, ISSN 0140-6736. DOI: 10.1016/s0140-6736(16)31321-6.
- ↑ a b c d e f g MARIAN, Ali J. – BRAUNWALD, Eugene. Hypertrophic Cardiomyopathy. Circulation Research. 2017, y. 7, vol. 121, p. 749-770, ISSN 0009-7330. DOI: 10.1161/circresaha.117.311059.
- ↑ PALEČEK, T – KUCHYNKA, P. , et al. Nesarkomerické formy hypertrofické kardiomyopatie v dospělosti. Kardiologická revue - interní medicína. 2011, y. 13, vol. 4, p. 210-220, ISSN 2336-2898.