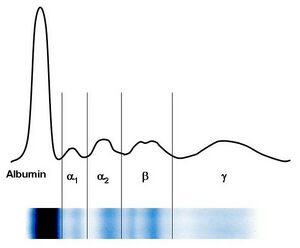
Serum protein electrophoresis
One of the basic screening examinations is serum protein electrophoresis.
Principle of serum protein electrophoresis[edit | edit source]
Electrophoresis (ELFO) is based on the movement of charged particles in an electric field. The determined substances must have the character of ions or ampholytes. Proteins are ampholytes that can acquire a positive or negative charge depending on the pH of the buffer in which the electrophoresis takes place. If a mixture of charged particles is exposed to an electric field, the molecules of the substances begin to move. Protein mobility is affected by the following factors:
- character of the divided substance (size of charge, shape and size of molecules, relative molecular weight);
- properties of the environment in which the division takes place (pH value, ionic strength, voltage, current).
Positively charged protein molecules are better adsorbed than negatively charged molecules, which is why negative charges are used in protein electrophoresis. The isoelectric point of most serum proteins is in the weakly acidic region at pH 5–6. Therefore, in an alkaline buffer environment, most often pH 8.6, serum proteins will be dominated by negative charges and their movement will be towards the anode with varying speed. Albumin exhibits the highest negative charge and thus the fastest mobility to the anode.
Electrophoresis can take place on different carriers. Historically, chromatography paper was the oldest. In clinical-biochemical practice, we most often encounter electrophoresis on cellulose acetate sheets or agarose gel. In electrophoresis, which uses paper or cellulose acetate as a carrier, protein molecules travel through a buffer solution that is outside the carrier. Molecules are therefore divided primarily according to their charge. Proteins will divide similarly even in a low-concentration agarose gel.
Using electrophoresis, blood serum proteins are usually divided into 5-6 fractions - albumin, α1, α2, β, γ fractions are sometimes distinguished into β1 and β2. With the exception of albumins, the other fractions are always made up of a group of proteins that have approximately the same electrophoretic mobility.
Execution[edit | edit source]
A drop of serum is added to a slide with an electrophoretic agorose gel and spread along the "start line", perpendicular to the direction of the future electric field. It is then exposed to the effects of an electric field in an electrophoretic bath. Under the influence of the electric field, the proteins begin to migrate in the agarose gel.
After a certain period of time (e.g. 30 minutes at a voltage of 120 V), the proteins in the gel are denatured ("fixed"), usually by the action of alcohols (methanol) and acids (acetic acid). This prevents them from being diffused or washed out of the gel in subsequent steps. The proteins are then stained with a suitable organic dye (e.g. amido black). The position of the individual fractions and the concentration of proteins in them are then evaluated using densitometry.
- You can find more information about electrophoresis in the form of an online video, for example, on the website Masaryk University.
Serum protein electrophoresis evaluation[edit | edit source]
| Faction name | Values in relative % | Values in g/l |
|---|---|---|
| Albumin | 55–69 | 35–44 |
| α1 | 1.5–4 | 1–3 |
| α2 | 8–13 | 5–8 |
| β | 7–15 | 4–10 |
| γ | 9–18 | 5–12 |
Characteristics of individual factions[edit | edit source]
- Zone of prealbumin
Prealbumin (transthyretin) moves in this area, but its zone is very discrete and difficult to evaluate.
- Albumin zone
Albumin creates a relatively wide and well-defined zone on both sides. When the albumin concentration falls below 30 g/l, its weakening is noticeable. The rarely observed doubling of the fraction is a manifestation of a genetic structural deviation of albumin in heterozygotes - bisalbuminemia or it arises when a foreign substance binds to albumin, e.g. penicillinu.
- Interzone between albumin and α1-globulins
The weak homogeneous staining of this area is due to α1-lipoproteins. Similar electrophoretic mobility is also shown by α1-acid glycoprotein, but it minimally affects the coloring of the zone.
- Zone of α1-globulins
The zone of α1-globulins is mainly affected by α1-antitrypsin. In acute inflammations, there is a clear increase. Clinically significant is the genetic variability of α1-antitrypsin, which in electrophoresis can often be clearly manifested by the weakening, sometimes even the disappearance of its zone with a simultaneous change in mobility.
- Interzone between α1 and α2-globulins
It is usually all weakly homogeneously colored.
- Zone α2-globulins
Two proteins are mainly involved in the formation of this zone - α2-macroglobulin and haptoglobin. Changes in α2-macroglobulin concentration are not of great diagnostic significance. Haptoglobin produces 6 phenotypes that differ in electrophoretic mobility. Electrophoretic examination does not allow distinguishing haptoglobinu phenotypes.
- Interzone between α2 and β1-globulins
Normally, it stains only weakly. During hemolysis, hemoglobin-haptoglobin complexes are formed, which create a separate zone in this area.
- Zone of β1-globulins
The shape and intensity of the staining of the β1-globulin zone is influenced practically only by transferrin. The intensity of the zone correlates well with the total binding capacity of the plasma for iron. In iron deficiency anemia and during pregnancy, the synthesis of transferrin increases and the intensity of the zone increases. Another protein with β1-electrophoretic mobility, hemopexin, is poorly stained by the dyes used, and changes in its concentration are not reflected in electrophoresis.
- Interzone between β1 and β2-globulins
In this area we find immunoglobulin IgA, which conditions the homogeneous coloring of the zone. Furthermore, it creates a typical β-lipoprotein line, the presence of which depends on its concentration.
- Zone of β2-globulins
The C3 component of complementu participates in the formation of the β2-globulin zone. It is difficult to estimate the concentration of C3 based on the color intensity.
- Zone of γ-globulins
The character of the γ-globulin zone is influenced by the concentrations of the four subclasses of immunoglobulin IgG. An increase in IgG will be manifested by a more intense coloration and an expansion of the zone in both directions. Immunoglobulin IgM lies closer to the start. A separate or simultaneous increase in IgM concentration cannot be detected by electrophoresis.
Clinical Use[edit | edit source]
Electrophoresis of serum proteins (ELFO) is performed especially if we find a pathological result of total protein or if we need more detailed information about serum proteins. The following is especially valuable for the card:
- dysproteinemia - change in the concentration and qualitative composition of individual proteins in the serum,
- paraproteinemia - characterized by the presence of monoclonal immunoglobulins.
ELFO in some pathological conditions[edit | edit source]
| Electrophorogram type | Comment | album | α1 | α2 | β | γ | Occurrence (example) |
|---|---|---|---|---|---|---|---|
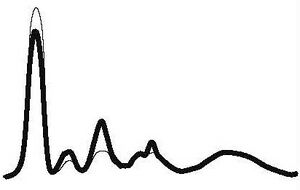
| Acute Inflammation |
|
|
| ||||
| ↓ or N | ↑ | ↑ | N | ||||
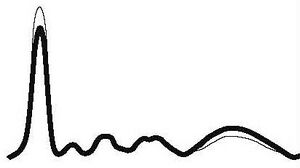
| Chronic Inflammation |
|
|
| ||||
| ↓ or N | N | N | N | ↑ | |||
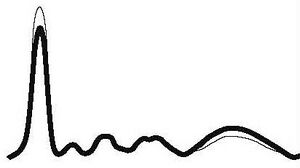
| Chronic active inflammation |
|
|
| ||||
| ↓ | ↑ | ↑ | N | ↑ | |||
| Hepatic type |
|
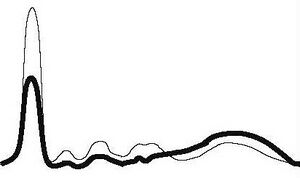
|
| ||||
| ↓ | ↓ | ↓ | ↓ | ↑ | |||
| Nephrotic type |
|
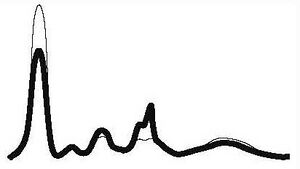
|
| ||||
| ↓ | N | ↑ | ↑ | ↓ or N | |||
| Hypogammaglobulinemia' |
|

|
| ||||
| N | N | N | N | ↓ | |||
| Monoclonal gammopathy |
|

|
| ||||
| ↓ | ↓ | ↓ | ↑ | ↑ | |||
Links[edit | edit source]
Related Articles[edit | edit source]
External links[edit | edit source]
![]() Video about electrophoretic methods used in clinical biochemistry for the examination of proteins in serum and urine
Video about electrophoretic methods used in clinical biochemistry for the examination of proteins in serum and urine