Electrophoresis
Electrophoresis is one of the separation methods that isolates molecules of different weight, or different electric charge, using their different mobility in the electric field.
From history[edit | edit source]
Since 1892 , it has been known that inorganic particles in a colloidal solution travel non-randomly under the action of an electric field. Not long after, this phenomenon was also described for proteins in aqueous solutions.
In 1948 , the Swedish scientist Arne W. Tiselius received the NC for chemistry for the assembly of the electrophoretic apparatus .
Principle of electrophoresis[edit | edit source]
Electrophoresis uses the ability of charged particles to move in an electric field, with the speed of particle movement depending on the size of the total surface charge, the size and shape of the molecule and its concentration in the solution.
The speed of a molecule during electrophoretic separation can be expressed as:
where ζ is the electrokinetic potential (V), ν is the linear speed of particle movement (m ·s−1), E is the electric field intensity (V · m−1) and η is the viscosity of the medium (Pa · s), εr is the relative permittivity liquids, ε0 vacuum permittivity, C a constant depending on the shape of the particles and the thickness of the electric double layer (e.g. for spherical particles with radius ra a large effective thickness of the double layer l, where r/l < 0.1, C = 2/3, for thin bilayer (r/l > 100) is C = 1).
Applications of electrophoresis[edit | edit source]
Free electrophoresis[edit | edit source]
Free electrophoresis is performed in aqueous solutions (electrolytes), in which the particles travel to an electrode with the opposite polarity. The separation can be disturbed by the influence of conventional currents arising from the influence of heat generated by the passage of an electric current.
Electrophoresis on carriers[edit | edit source]
It is carried out on hydrophilic porous supports, such as uncoated (chromatographic) paper, cellulose acetate, agarose gel, polyacrylamide gel, cellulose, non-gelling starch, etc. Depending on the material, the support can act as a molecular sieve through which large molecules pass more difficult than small ones.
Zonal electrophoresis of plasma proteins[edit | edit source]
Zonal, also zonal, electrophoresis is performed, for example, on cellulose acetate. The division of proteins here depends on the type and number of ionizable groups of amino acid side chains and on the size of the total charge of the protein (positive or negative).
If pH of the solution in which the electrophoresis takes place is greater than the pI (isoelectric point), the protein has an overall negative charge. Conversely, if pH of the solution is lower than the pI, the protein has an overall positive charge. If pH of the solution corresponds to the isoelectric point of the protein, it does not move.
Gel electrophoresis[edit | edit source]
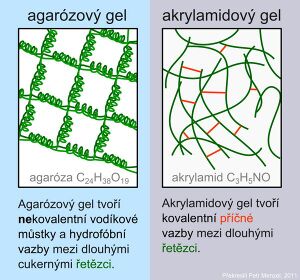
Gels form the transition between solid and liquid states. Structurally, the gel is more than 90% water. In the gel, we find a three-dimensional network where the pores can serve as a molecular sieve. The size of the pores corresponds to the size of proteins and nucleic acids. The most common gels are polyacrylamide and agarose.
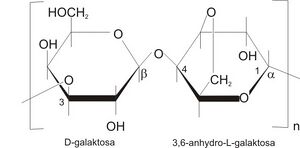
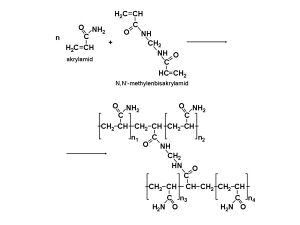
Agarose is a network formed by long sugar polymers linked by non-covalent hydrogen bonds and hydrophobic bonds. Polyacrylamide gels form a network of long polyacrylamide molecules connected by cross-bridges of N,N'-methylenebisacrylamide.
Native protein gel electrophoresis[edit | edit source]
It takes place without denaturing agents. Proteins migrate through the gel according to their overall charge, size, and shape. The sensitivity of electrophoresis is determined by the nature of the pores of the gel. An example would be serum protein electrophoresis .
SDS gel electrophoresis[edit | edit source]
Proteins are denatured with sodium dodecyl sulfate (SDS) and β-mercaptoethanol, which breaks the disulfide bonds. SDS is a denaturing agent and detergent that coats and denatures proteins. The proteins acquire a rod-like shape and at pH 7–10, they all have the same surface charge density due to SDS. Their mobility depends almost exclusively on the molecular weight of the polypeptide chains. The method is suitable for the analysis of macromolecular complexes.
Isoelectric focusing[edit | edit source]
Proteins are separated in a pH gradient gel. They migrate to the point where they have no surface charge, i.e. the point where pH equals pI.
Two-dimensional protein electrophoresis[edit | edit source]
2D electrophoresis enables resolution of up to 10,000 proteins. Proteins are first separated according to their isoelectric points in a pH gradient. After separation in the first dimension, SDS-electrophoresis is performed in the second direction, the proteins are separated according to their size.
Unlike previous methods, which result in fractions often containing a mixture of several proteins, 2D electrophoresis makes it possible to isolate individual proteins even from biological samples. The resulting protein maps can be compared with control samples (eg patients with a specific disease and healthy patients). 2D electrophoresis is also used as a preparative method: a specific spot is cut out of the gel, the protein is isolated from it and used for further analysis or another procedure.
Capillary electrophoresis[edit | edit source]
The method uses the movement of electrically charged substances in an electric current dissolved in a conductive medium - capillaries filled with electrolyte or gel. An accompanying phenomenon is electroosmotic flow. An electrical double layer forms on the inner surface of the capillary at the point of contact with the conductive solution. The fixed part (wall of the capillary) is charged with an immobile surface negative electric charge. From the liquid part, the positive ions attach to the capillary wall (double layer) and the ions move to the cathode. The moving layer moves through the capillary and takes with it the entire cross-section of the liquid in the capillary. The solution travels through the capillary all at once (with electrophoresis, only ions travel)
Using electrophoresis[edit | edit source]
Electrophoresis is widely used, for example in the analysis and separation of protein mixtures, characterization of the surfaces of organisms such as bacteria, viruses etc. Practical use in the diagnosis of monogenic diseases etc.
Links[edit | edit source]
Related Articles[edit | edit source]
- Serum protein electrophoresis
- Electrophoresis of nucleic acids
- Separation of DNA fragments by electrophoresis
References[edit | edit source]
- MURRAY, Robert K, et al. Harper's Biochemistry. 2nd edition. Jinočany: H&H, 2002. 872 pp. ISBN 80-7319-013-3
- Wikipedia. Electrophoresis [online]. ©2012. Last revision 2012-01-30, [cit. 2012-04-17]. < https://cs.wikipedia.org/wiki/Elektrofor%C3%A9za >
- ALBERTS, B, D BRAY and A JOHNSON, et al. Basics of cell biology. 2nd edition. Ústí nad Labem: Espero Publishing, 2005. 740 pp. ISBN 80-902906-2-0