Skin cancers
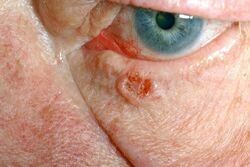
Basalioma[edit | edit source]
It is the most common malignant skin tumour. Its incidence has doubled in the last 15 years.
Incidence[edit | edit source]
Increased incidence is observed after 40 years of age, with the over 60 group being the most at risk. However, it can occur at any age. It affects almost exclusively white individuals; when it occurs in pigmented races, it is associated with unusual etiologic factors. The tumour arises from keratinocytes of the epidermis.
Clinical picture, evolution[edit | edit source]
The typical cells are oval in shape and resemble cells of the basal layer of the epidermis - hence the name. The tumor almost never metastasizes - so far, over 200 cases of metastasis have been described worldwide, mostly to the lymph nodes. It usually starts as a nodule, vesicle or stroma which does not change significantly initially. It grows slowly, as if crawling on the surface. It consists of a single solitary nodule with raised margins, parts of which may undergo swelling that does not heal. Sometimes there is deceptive improvement, the ulcer almost heals, only to reappear shortly afterwards and slowly enlarge. The inconspicuous course often results in the formation eventually enlarging to a size that can be a therapeutic problem in some localisations. On the other hand, basaliomas on mucous membranes metastasize very frequently.
Cause of the disease[edit | edit source]
As with other malignant diseases, the cause of the disease is unclear. The main etiologic factor is chronic, long-term exposure of the skin to UV radiation.
Prognosis[edit | edit source]
With the exception of large or invasive tumors, basalioma is not life-threatening to the patient. However, if the tumor grows long enough, it can form large foci with destruction of adjacent tissues.
Treatment[edit | edit source]
Surgical removal. If it is not excised whole, it will recur! It then needs to be excised until the entire lesion is removed. Due to the site of occurrence (face), the aesthetic aspect of the procedure cannot be neglected.
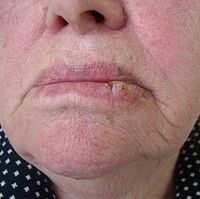
Spinalioma[edit | edit source]
Also squamous cell carcinoma, is classified as a malignant epithelial tumor of the skin. It usually begins with intraepithelial growth, followed by destructive progression. It metastasizes predominantly via the lymphatic route. The incidence in our population corresponds to about 11/100 000, but it is less frequent (about 1:10) compared to basalioma.
Etiology[edit | edit source]
It usually develops from precancerous lesions (solar keratosis, leukoplakia, m. Bowen, etc.), especially in predisposed individuals, with lower amounts of melanin in the skin (phototype I and II). Other risk factors are chronic degenerative skin changes (scar, fistulae, skin ulcer), immune disorders (immunosuppression), HPV infection and long-term exposure of the skin to carcinogens.
Clinical presentation[edit | edit source]
The clinical picture of the tumour evolves over time.
- form: Diffuse infiltrating (inconspicuously raised hyperkeratosis or a solid, infiltrating lesion with a bumpy surface, grows slowly, metastasizes late);
- form: Ulcerative (may break down in the centre and develop an ulcer with rolled, rigid margins);
- form: Exophytic (soft, aggressive and rapidly growing mass with central breakdown and haemorrhage, metastasises early).
Metastasis to regional nodes occurs in about 5-10% of patients. Nodules tend to be solid, large in size, with the possibility of ulceration and fistula formation.
Special forms with their typical precancerous appearance are distinguished:
- Spinalioma of the lip (from leukoplakia and cheilitis);
- Spinalioma of the vulva (from lichen sclerosus et atrophicus);
- spinalioma of the penis (from erythroplasia);
- spinalioma of the tongue (from leukoplakia and erythroplakia);
- aggressively growing exophytic form (from m. Bowen and chronic irritation).
Diagnostics[edit | edit source]
The decisive examination for the diagnosis is the histopathological examination. The prognosis of the tumor correlates with the degree of cell dedifferentiation.
Therapy and prognosis[edit | edit source]
Radical excision with a rim of healthy tissue (peripheral and deep). For smaller lesions, an intact margin of about 1 cm width is recommended. Excision of larger tumours should have a healthy tissue margin of 2-3 cm, especially on the trunk, where the spinalioma behaves more aggressively and resolution of the defect does not cause major difficulties. In case of metastases to regional nodes, we also perform their removal.
If surgery is not possible (e.g. in elderly people), we perform radiotherapy. In the presence of metastases, we add chemotherapy.
The prognosis depends on the location, size of the tumour and the degree of dedifferentiation. Best prognosis is for tumours in the solar region, worse for tumours in the ear, lips and scars.
Melanoma[edit | edit source]
Malignant melanoma is a cancer that arises from neoplastic proliferation melanocytes. It is classified as a neuroectodermal tumour. Malignant melanoma predominantly affects the skin but can also affect the eye, ear, leptomeninges, GIT (histology) and the mucous membranes of the mouth or genitalia. The incidence of melanoma is increasing, affecting mainly the white population.
Melanoma occurs in four basic histological types: superficial spreading melanoma, lentigo maligna melanoma, acrolentiginous melanoma and nodular melanoma. The mainstay of therapy is surgical resection of the tumour together with a sufficient margin of adjacent skin, or resection of the lymph nodes. Adjuvant treatment with interferon α, specific vaccines, BRAF inhibitors (Vemurafenib) and CTLA-4 blockers (Ipilimumab) are considered.
Epidemiology[edit | edit source]
The incidence of melanoma is increasing worldwide. In the white population in the United States, the incidence of melanoma has more than tripled over the past 20 years. The incidence of melanoma shows geographic variation. While in North America there are 6.4 new cases per 100,000 men and 11.7 new cases per 100,000 women, in Australia and New Zealand there are already 37.7 cases per 100,000 men and 29.4 cases per 100,000 women.[1] Incidence of melanoma in Czechia was in 2018 18,4 per 100 000 men and 15,6 per 100 000 women.[2]
Malignant melanoma accounts for approximately 4% of all skin cancers but is responsible for up to 73% of skin cancer deaths. Worldwide, survival is higher in developed countries (91% in the US, 81% in Europe) than in developing countries (approximately 40%). The lower mortality in developed countries is mainly due to greater public awareness, leading to earlier diagnosis and treatment.
Malignant melanoma primarily affects the white population. The prevalence of melanoma in the Hispanic population in the United States is approximately six times lower than in the white population. In African Americans, the prevalence is as much as 20 times lower. [Mortality]] Hispanics and African Americans from malignant melanoma is, however, higher than that of the white population. This is due to the higher incidence of acral melanoma and the more advanced state of the disease.[3]
Up to 39 years of age, melanoma is more common in women, from 40 years of age it is more common in men. Overall, women are slightly more affected (male to female ratio, 0.97:1), but mortality is higher in men.[3]
The Median of melanoma diagnosis is 59 years, however melanoma is the most common type of cancer in women between 25-29 years of age and between 30-34 years of age it is the second most common type of cancer after breast cancer. Malignant melanoma affects more older individuals, who are also more likely to succumb to the disease. Therefore, the elderly should be the main target for secondary prevention of melanoma.[3]
Pathophysiology[edit | edit source]
The pathophysiology of melanoma development is not fully understood. Multiple pathogenetic mechanisms of melanoma development are hypothesized. Melanoma develops not only on sun-exposed skin, where UV radiation is the main pathogenetic factor, but also in areas that are relatively protected from radiation (the trunk).[1]
Malignant melanoma is accompanied by mutations in the BRAF, NRAS and KIT genes. Individual mutations are dependent on the mode of exposure to sunlight. While the BRAF mutation occurs more in intermittent sun exposure and is more common in superficial spreading melanoma, the KIT mutation occurs more in chronic exposure or on relatively unexposed skin and is more common in nodular melanoma.
Melanoma arises either from malignancy of an existing melanocytic nevus or more commonly de novo (in more than 70% of cases).[1]
The risk factors include.
- age over 50 years, [4]
- higher sensitivity to sunlight,
- excessive exposure to sunlight in childhood, bullous dermatitis after childhood sunburn,
- increased number of atypical (dysplastic) nevi [1]
}}</ref> or large congenital nevus (greater than 20 cm in adulthood), [4]
- a family history of melanoma,
- presence of a changing pigmented patch, [1]
- immunosuppression,
- tanning bed use. [4]
Clinical picture and histopathological types[edit | edit source]
Melanoma is most commonly found on the trunk in Caucasian males, whereas in Caucasian females it is most commonly found on the tibia or back. Melanomas in Hispanics, African Americans, and Asians most commonly occur on the plantar region, followed by subungual localization and palms and mucous membranes.
Melanoma can affect the skin as well as the mucous membranes (e.g. oral) or the eye.[5]
Cutaneous melanoma is divided into four basic clinicopathological subtypes according to the nature of growth, anatomical location of the lesion and the degree of sun damage:
Superficial spreading melanoma[edit | edit source]
This subtype represents approximately 70% of cutaneous melanomas and is the most common subtype of melanoma in individuals between 30-50 years of age.[5]
The tumor initially behaves as a carcinoma in situ that grows radially and is not prone to metastasize. At this stage, melanoma can persist for months or years. It occurs most commonly on the trunk of men or on the tibia of women. It arises from intermittent sun exposure.[5]
It often presents with the warning signs of ABCD (see diagnosis). It is either flat or slightly raised. Usually larger than 6 mm. Histologically, there is a pagetoid distribution of atypical melanocytes in the epidermis (similar to the scattered shotgun pellets after a shotgun blast, the so-called "buckshot scatter").[5]
Nodular melanoma[edit | edit source]
Represents 15-30% of cutaneous melanomas.[5]
Nodular melanoma lacks the carcinoma in situ stage, grows relatively rapidly, and invades deeper skin structures. The most common location is the legs and trunk of both men and women. It arises from intermittent sun exposure.[5]
It usually does not manifest with ABCDE warning signs and can easily escape attention. It usually manifests as a brownish-black papule or even nodular formation that ulcerates and bleeds easily. In addition, the lesion may be amelanocytic, i.e. without pigment.[5]
Lentigo maligna melanoma[edit | edit source]
The incidence of lentigo maligna melanoma is increasing. It mainly affects older individuals around 65 years of age.[5]
Lentigo maligna is a carcinoma in situ with radial growth with no tendency to metastasize. The lesion is usually larger in diameter (1-3 cm) with dark brown to black pigmentation, although hypopigmentation is also not uncommon. At this stage, lentigo maligna persists for at least 10-15 years before dermal invasion and the development of lentigo maligna melanoma, which forms a raised blue-black nodule on the surface. The typical localization is the head, neck and arm. It occurs with chronic sun exposure.[5]
Acrolentiginous melanoma[edit | edit source]
The least common subtype of melanoma in white populations, but the most common subtype of melanoma in dark-skinned populations (African Americans, Hispanics, Asians).[5]
The tumor initially behaves as a carcinoma in situ that grows radially and is not prone to metastasize. At this stage, melanoma can persist for months or years. It is most often found on the palms, soles or under the nail plate (subungual melanoma). The spread of pigment to the proximal or lateral nail bed is referred to as Hutchinson's sign, typical of subungual melanoma. Acrolentiginous melanoma is not related to sun exposure like mucosal melanomas.[5]
Differentially, one should think of benign junctional melanocytic nevus, subungual hematoma and onychomycosis.[5]
Rare subtypes of cutaneous melanoma[edit | edit source]
They occur in less than five percent of cases.
- Desmoplastic melanoma is a rare but important subtype of cutaneous melanoma that occurs predominantly in older individuals (60-65 years of age). It mainly affects the sun-exposed skin of the head and neck. Clinically, it is more similar to non-melanocytic skin tumours and may therefore cause a delay in diagnosis. Desmoplastic melanoma often has perineural spread and tends to recur locally. Radical excision with adjuvant radiotherapy is therefore recommended for therapy.
- Mucinous (lentiginous) melanoma.
- Malignant blue nevus.
- Melanoma arising from a large congenital nevus.
- Clear cell sarcoma.
- Amelanocytic melanoma is pigmentless and can occur simultaneously with any subtype of melanoma (usually nodular or desmoplastic). It may mimic basal cell or squamous cell carcinoma, dermatofibroma or even hair follicle lesions.[5]
Diagnostics[edit | edit source]
The most common warning sign of melanoma is a newly formed and changing pigmented spot. Symptoms such as bleeding, itching, ulceration or pain at the site of the pigment spot may occur. For clinical purposes, the ABCDE rule has been developed to assess the warning signs of melanoma:
- A - Asymmetry - the pigment spot is not symmetrical.
- B - Border irregularity - the edges of the spot are uneven, jagged or indistinct.
- C - Color variegation - the coloration is not uniform, showing different shades of skin color, brown and black. White, red, or blue pigment spots tend to be a worrisome finding.
- D - Diameter - a diameter greater than 6 mm is characteristic of melanoma, although smaller diameters may occur. Any growth of a spot merits examination.
- E - Evolution - the pigmented spot changes over time. This point is especially important in nodular melanoma or amelanocytic melanoma (without pigment), which may lack ABCD points.
Lesions that show these characteristics are evaluated as potential melanoma.[6]
It is practical for the clinician to evaluate the ugly duckling warning sign. This is such a pigmented spot that somehow distinguishes itself from others. It is useful to combine this sign with the ABCDE criteria.[6]
When melanoma is suspected, it is important to biopsy the suspicious lesion on the skin or mucosa (excision with a 1-3 mm margin of healthy tissue) and subsequent histological examination. The biopsy report then provides five basic pieces of information:
- Tumor thickness in millimeters (Breslow)[7] - is the most important prognostic factor, measured from the upper border of the stratum granulosum to the deepest point of tumor invasion. The greater this thickness, the higher the potential for metastasis and therefore the worse the prognosis.[8]
- Ulceration [7] – is second most important prognostic factor.[8]
- Skin mitosis number – number of mitosis per 1/mm2 increases probability of metastasizing .[8]
- Microsatellitosis'[7]
- Anatomic grade of invasion (Clark) - only for tumors less than or equal to 1 mm and when mitotic index cannot be assessed; optional for tumors greater than 1 mm.[7] According to statistics, Breslow, ulceration, mitotic index, age, gender or lesion location do not have the same prognostic significance for patient survival.[8]
When histology is unclear, immunohistochemical staining may aid diagnosis, for example for S-100, HMB-45 (homatropine methylbromide 45), melan-A/Mart-1 or markers of proliferation such as Ki67 (proliferating cell nuclear antigen).[7]
If the tumor is larger than (or equal to) 1 mm or if a thinner tumor shows unfavorable prognostic factors such as ulceration or a higher number of mitoses, a sentinel node biopsy is performed to help determine disease staging.[9]
Elevated levels of serum LDH in biochemical examination are a negative prognostic marker and are associated with shorter survival.[9]
Indications for imaging tests such as X-ray, CT, MRI or PET are considered on an individual basis, for example to assess the presence of metastases.[9]
Staging[edit | edit source]
Melanoma is divided into stages 0-IV according to histology:
- Stage 0 is carcinoma in situ.
- Stage I and II have depth of invasion as a criterion.
- Stage III affects regional lymph nodes.
- Stage IV represents distant metastases in the skin, subcutaneous tissue, nodes, visceral, skeletal or CNS.
In addition, individual stages have subgroups according to the presence of various features, such as the presence of ulceration, the number of mitoses, or LDH (lactate dehydrogenase) levels. For example, a lesion 2.01-4 mm wide without ulceration is rated as stage IIA, but the same lesion with ulceration is rated as stage IIB.[10]
Therapy[edit | edit source]
Surgery[edit | edit source]
The primary method of therapy for the localized form of malignant melanoma is surgical resection of the tumor deposit with a sufficiently wide margin of healthy skin. For carcinoma in situ, this margin is 5 mm wide. For low-risk melanomas of depth (Breslow) 1 mm or less, a rim 1 cm wide is recommended for excision. For melanomas 1 mm or more in depth, a 2 cm border is recommended. Studies have shown that a margin wider than 2 cm does not provide clinical benefit.[11]
Selective lymph node dissection can improve the prognosis of patients with malignant melanoma, according to a WHO study.[11]
Previously, the problem of whether to perform regional lymphadenectomy for melanomas 1 mm or more in depth (Breslow) or less than 1 mm but with negative prognostic features such as ulceration, lymphovascular invasion, or a higher number of mitoses was addressed. In the 1990s, the use of sentinel node biopsy (SLNB) methods for malignant melanoma began, which solved this problem.[11] For sentinel node imaging, 99mTc is injected peritumorally. The radiopharmaceutical is drained into the sentinel lymph node by lymphatics. Peroperatively, this lymph node can be identified with a gamma camera. The second option is the application of dyes such as methylene blue or lymphazurin, which stain the lymph node. When both methods are combined, the probability of finding a sentinel node is approximately 95% (influenced by anatomical location).[12]. The sentinel node thus localized is removed and examined histologically or immunohistochemically for the presence of micrometastases. If the biopsy specimen is positive, a complete lymph node dissection (CLND) is performed. If the findings are negative, the nodes are retained.[11]
Adjuvant therapy[edit | edit source]
Adjuvant therapy in the form of chemotherapy (dacarbazine therapy), radiotherapy, biological therapy, non-specific immunomodulation or vitamin therapy has no effect on patient survival and is still under investigation.[13]
One hope may be high-dose interferon α therapy, which statistically reduces the incidence of relapse. The disadvantage is the long-term high-dose therapy and the resulting side effects. These may include flu-like symptoms, manifestations of intolerance to therapy or induction of autoimmunity with the production of autoprotective antibodies (antithyroid, antinuclear, anticardiolipin). The prognostic significance of induced autoimmunity in malignant melanoma is still under study.[13]
A second hope may be the administration of specific vaccines that contain melanoma antigenic structures. These vaccines, as we might expect, are not of value in prevention but act as a stimulator of the immune system in advanced forms of the disease (St. III, IV). Unlike interferon therapy, it does not have as many side effects. The efficacy of the vaccines alone has not been proven, but the combination of a peptide vaccine (gp100:209-217(210M)) with high-dose IL-2 therapy has shown good results so far.[13]
The third avenue of research is the so-called BRAF-inhibitors (Vemurafenib), i.e. inhibitors of some mutated forms of serine-threonine kinases. Vemurafenib therapy significantly reduces the risk of disease progression and prolongs survival. However, further studies need to complete this pathway.[13]
For metastatic or unresectable melanoma, Ipilimumab, a monoclonal antibody against cytotoxic T-lymphocyte antigen 4 (CTLA-4), may be used. Blockade of CTLA-4 results in enhanced T-lymphocyte activity and proliferation. The actual antitumor mechanism of action is nonspecific, mediated by T-lymphocyte activity.[14]
As can be seen from the previous text, the diagnosis and therapy of malignant melanoma depends on interdisciplinary collaboration. It involves a dermatologist, a pathologist, a surgeon, a nuclear medicine specialist, an oncologist, or a radiation oncologist.[15]
Patients diagnosed with malignant melanoma should be dispensed for risk of recurrence. Most often, metastases occur 1-3 years after treatment of the primary tumor. In 4-8% of patients with a history of malignant melanoma, a new primary melanoma lesion develops within 3-5 years.[16]
Kaposi's sarcoma[edit | edit source]
Kaposi's sarcoma is a mesenchymaleral malignant vascular tumor.
Etiology[edit | edit source]
An association with HHV-8 has been demonstrated. It occurs in immunosuppressed patients.
There are 4 variants of Kaposi's sarcoma:
- classic form (lower limbs of elderly people);
- Iatrogenic form in immunosuppressed patients after cytostatic treatment, e.g. after transplantation;
- endemic form (Africa);
- epidemic form in patients with AIDS'.
Clinical picture[edit | edit source]
Classical form[edit | edit source]
Stiff livid slow-growing bumps of deep red, later to brown in color. Localization around the ankles and on the shins. Risk of exulceration and bleeding. Typically, diffuse oedema is associated.
Disseminated form[edit | edit source]
Occurs anywhere on the trunk and mucous membranes. Most commonly associated with AIDS. Affects internal organs in addition to the skin. The cutaneous manifestations are very varied. It is very aggressive and progresses rapidly.
Diagnosis[edit | edit source]
Histopathology. Image of atypical capillaries in the corium. The slit-like spaces are filled with spindle cells filled with erythrocytes.
Diff. diagnosis[edit | edit source]
- Pseudo-Kaposi's sarcoma - occurs in CHD,
- Hemangioma,
- Hemangiosarcoma.
Therapy[edit | edit source]
For the classic form, primarily surgical. For systemic involvement, mainly AIDS treatment, also interferon-α, chemotherapy.
Prognosis[edit | edit source]
In the classical form, good. In general disability, fatal.
Dermatofibrosarcoma protuberans[edit | edit source]
Dermatofibrosarcoma protuberans is a malignant connective tissue tumor characterized by local invasion and frequent recurrence. The tendency to metastasis is low.
Clinical picture[edit | edit source]
It occurs as a solid lesion on the upper body. During development, it forms as painful brownish-red or bluish bumps with vascular ectasia on the surface. Infiltration of the lower layers of skin is relatively common. It rarely metastasizes to regional lymph nodes or lungs.
Diagnosis[edit | edit source]
Histopathologically, it contains CD34+ fibroblasts in a typical storiform (mat-like) arrangement.
Differential diagnosis[edit | edit source]
Therapy[edit | edit source]
Surgical removal with a wide margin of min 3-5 cm into healthy tissue and deep to the fascia.
Prognosis[edit | edit source]
Chronic course, frequent recurrences after surgical treatment. Rarely metastasizes.
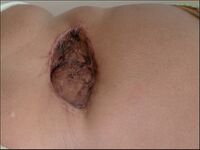
Verrucous carcinoma[edit | edit source]
Verrucous carcinoma (Carcinoma verrucosum) is a highly differentiated variant of squamous cell carcinoma of the skin, characterized by slow growth and warty surface.
It is one of the invasive carcinomas but has a very low tendency to metastasis.
Epidemiology[edit | edit source]
It occurs mainly in elderly patients in areas with scarring, chronic wounds and in chronic venous insufficiency. In some cases, an association with HPV 6 and 11 has been demonstrated.
Clinical picture[edit | edit source]
Four forms are distinguished, mainly according to localization.
| Clinical picture | ! Occurrence | |
|---|---|---|
| 1. Epithelioma cuniculatum | A large cauliflower-shaped mass with tunnels and crypts, later oozy material can be extruded from the mass. Damage to the metatarsal bones may occur. | Most commonly in old men on the soles of the feet. |
| 2. Papillomatosis cutis carcinoides (Cutaneous verrucous carcinoma) | Bordered lesions up to palm size with hyperkeratosis and cauliflower-like vegetation that does not heal for years. | On tibiae in places with CHŽI or around ulcers. |
| 3. Florida oral papillomatosis | Mucosal leukoplakic lesions with papillomatous surface. May extend to the mandible and cause difficulty eating and speaking. Predisposing factors include poor oral hygiene, alcoholism, tobaccoism. | Oral surface, lips, larynx. |
| 4. Huge condylomas (Buschke-Löwenstein) | Grey to pink sessile cauliflower-like formations. They are very invasive. . | On the penis, vulva and perianogenitally. |
Diagnosis[edit | edit source]
Histological. It is an asymmetrically proliferating mass into the epidermis. It contains cornified crypts.
Therapies[edit | edit source]
Surgical treatment is necessary. It is supplemented by radiotherapy, chemotherapy, laser destruction of the tumor, or general treatment with retinoids, interferons.
Links[edit | edit source]
Related articles[edit | edit source]
- Malignant skin tumors: Melanoma | Basal cell carcinoma | Verrucous carcinoma
- Benign skin tumors
- Pre-cancers in dermatology
- Malignant mesenchymal tumors: Kaposi's sarcoma | Dermatofibrosarcoma protuberans
- Kaposi's sarcoma
- Malignant skin tumors: Melanoma - Basal cell carcinoma - Squamous cell carcinoma - Verrucous carcinoma
- Benign skin tumors
- Pre-cancers in dermatology
External links[edit | edit source]
Literature used[edit | edit source]
- JANEČEK, Vladimír. Spinaliom [online]. [cit. 2011-02-09]. <<http://www.liposukce.cz/plasticka-chirurgie/kozni-nadory/spinaliom.htm>>.
- VOKURKA, Martin. Veľký lekársky slovník. 1. edition. Maxdorf, 2002. 923 pp. ISBN 80-85912-43-0.
- ŠTORK, Jiří. Dermatovenerologie. 1. edition. Galén, Karolinum, 2008. 502 pp. ISBN 978-80-7262-371-6.
- MĚŠŤÁK, Jan. Úvod do plastické chirurgie. 1. edition. Univerzita Karlova v Praze - Nakladatelství Karolinum, 2005. 125 pp. ISBN 80-246-1150-3.
- ↑ a b c d e SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ KRUŽICOVÁ, Zuzana. Maligní melanom [online]. [cit. 2012-03-09]. <https://zdravi.euro.cz/clanek/postgradualni-medicina/maligni-melanom-450829>.
- ↑ a b c SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ a b c SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ a b c d e f g h i j k l m SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ a b SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ a b c d e SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ a b c d SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ a b c SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ a b c d SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ Rogerio I. Neves. Increased Post–Operative Complications With Methylene Blue Versus Lymphazurin In Sentinel Lymph Node Biopsies For Skin Cancers [online]. [cit. 2012-03-09]. <http://200.40.135.66:90/toc/7574/2011/7574-20110401-5-10.pdf>.
- ↑ a b c d SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.
- ↑ SWETTER, Susan M. Cutaneous melanoma [online]. [cit. 2012-02-23]. <https://emedicine.medscape.com/article/1100753-overview>.