Overview of Energy Metabolism
Subchapter content
- Basic terms of energy metabolism.
- Energy metabolism.
- Position of organs in energy metabolism.
Basic terms of energy metabolism
Metabolism
Energy metabolism
Continuous regeneration of macroergic compounds is necessary for life. It serves as a source of free energy for the course of endergonic reactions. Their formation begins with the breakdown of high-molecular substances. These are then converted to basic intermediates, such as Acetyl-CoA. They are further oxidized during aerobic metabolism in the citrate cycle and the resulting reduced coenzymes (NADH + H+ and FADH2) are used in the respiratory chain to create ATP. (FBLT) }}
Macroergic compounds
Macroergic compounds are substances containing macroergic bonds, i.e. bonds that release large amounts of energy when they are cleaved.
ATP
ATP is a major and versatile macroergic compound. It provides partial storage but mainly free energy (G) transfer in the cell. In addition to ATP, there are also other macroergic compounds that are capable of releasing a larger amount of energy by splitting. ATP is used the most. This is due to the relative stability of the anhydride bond, which resists spontaneous hydrolysis, unlike other anhydrides, and is cleaved only in the presence of enzymes.
Substances with more negative free energy values can then be used to regenerate ATP, e.g. phosphoenolpyruvate, creatine phosphate, 1,3-bisphosphoglycerate. Macroergic compounds are substances containing macroergic bonds, i.e. those bonds that release large amounts of energy when they are cleaved.
Generation of ATP
ATP can be produced in a cell by:
- Phosphorylation at the substrate level. This is an energy coupling exergonic reaction with the synthesis of ATP from ADP and Pi. Three reactions are most often described: two are part of glycolysis (conversion of phosphoenolpyruvate to pyruvate and 1,3-bisphosphoglycerate to 3-phosphoglycerate), one is part of the Krebs cycle (conversion of succinyl-CoA to succinate).
- Aerobic phosphorylation and the respiratory chain.
 For more information see Respiratory Chain and ATP Generation (FBLT) .
For more information see Respiratory Chain and ATP Generation (FBLT) .
Energy release from ATP
ATP hydrolysis takes place in several steps:
- ATP → ADP + Pi (ΔG = −30.5 kJ/mol);
- There is one more macroergic bond in ADP that can be used. However, since the use of ADP instead of ATP is problematic due to the substrate specificity of the enzymes, the reaction catalyzed by ``adenylate kinase usually takes place:
- 2 ADP → ATP + AMP
- and the generated ATP is used.
- ATP → ADP + Pi (ΔG = −30.5 kJ/mol);
Diphosphate (pyrophosphate, PPi) is directly released from ATP by the action of some enzymes:
- ATP → AMP + PPi (ΔG = −45.6 kJ/mol).
PPi can be further cleaved by the enzyme ``diphosphatase (pyrophosphatase) to release energy.
Other macroergic compounds
- Other nucleoside triphosphates are less versatile and are used for specific purposes. E.g. UTP serves to activate carbohydrates for their entry into metabolic pathways.
- Enol phosphates contain a − OH group, which is esterically bound to the phosphate. The most important representative, phosphoenolpyruvate (PEP), is a macroergic compound with the highest energy potential ΔG (up to − 61.9 kJ/mol). Therefore, the reaction of converting PEP to pyruvate is also an irreversible reaction of glycolysis.
- Acyl phosphates contain an anhydride bond −COOH with phosphate. These include carbamoyl phosphate (used in the synthesis of urea) or 1,3-bisphosphoglycerate (an intermediate of glycolysis).
- Other macroergic compounds include guanidine phosphates' (eg creatine phosphate) or thioesters and thioethers (HS-CoA derivatives, SAM). Sometimes we can come across the term low-energy phosphates. Low-energy phosphates include, for example, glucose-6-phosphate and release less energy, between 9 and 20 kJ/mol.
Reference
Major intermediates of energy metabolism
In cells, there are metabolic pathways – a kind of crossroads of mutual transformation of nutrients. These include the ``pyruvate dehydrogenase reaction' (PDH), the ``Krebs cycle (KC) and the respiratory chain (DŘ). Three intermediates, acetyl-CoA, pyruvate' and NADH, have an important position in energy metabolism.
Acetyl-CoA
Creation
- Pyruvate dehydrogenase reaction (PDH)
- The irreversibility of this reaction is the reason that glucose cannot be formed from the vast majority of fatty acids.
- Degradation of amino acids
- Lysine and leucine are degraded directly to acetyl-CoA, other amino acids are converted via pyruvate.
- β-oxidation of fatty acids and degradation of ketone bodies.
Usage
- The Krebs cycle, which is followed by the respiratory chain (and ATP production).
- Synthesis of fatty acids and ketone bodies (with an excess of acetyl-CoA).
- Cholesterol synthesis.
Pyruvate
Creation
- Aerobic glycolysis.
- Lactate oxidation (catalyzed by lactate dehydrogenase).
- Degradation of some AKs.
Usage
- Synthesis of acetyl-CoA (PDH).
- Lactate synthesis – takes place during anaerobic glycolysis, the purpose of which is to regenerate reduced coenzymes NADH + H+ back to NAD+.
- Alanine synthesis (catalyzed by alanine aminotransferase).
- Synthesis of oxaloacetate (catalyzed by pyruvate carboxylase).
- Gluconeogenesis.
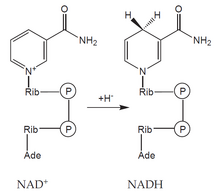
NADH
Creation
- Aerobic glycolysis
- Pyruvate dehydrogenase reaction.
- Beta-oxidation of fatty acids.
- Krebs cycle.
- Oxidation of ethanol.
Usage
- The respiratory chain and the formation of ATP.
- Conversion of pyruvate to lactate.
Link
Position of organs in energy metabolism
Liver
- Liver cells (hepatocytes) have a fundamental role in maintaining homeostasis, in the synthesis of molecules, in the mutual conversion of nutrients and in the regulation of energy storage and release. It participates in the metabolism of all nutrients.
Carbohydrate Metabolism
- In the metabolism of carbohydrates, their short-term , in the range of hours, and long-term , in the range of days to weeks, regulation of glycemia - glucostatic function of the liver is important. With a high level of glucose in the vena portae after a meal, glycogen synthesis is started in the liver , which consumes the glucose taken from the blood. On the contrary, during fasting and a drop in blood glucose levels, glucose is added to the circulation through glycogenolysis – the breakdown of stored glycogen, or, when glycogen stores are depleted, gluconeogenesis .
Lipid metabolism
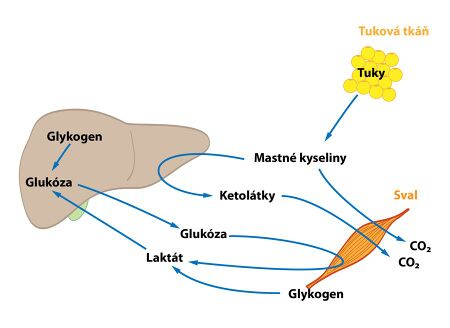
- Some pathways of lipid metabolism are unique to the liver - the synthesis of ketone bodies. Most of the pathways also occur elsewhere, but they are quantitatively most significant in the liver. Oxidation of fatty acids takes place here . During starvation, this pathway runs to a greater extent than the liver needs to produce energy for its own consumption. From the resulting acetyl-CoA , ketone bodies are subsequently formed, which the liver cannot process on its own, so they release them into the circulation, where they serve as an alternative source of energy. Cholesterol synthesis also takes place in the liver.
- The position of the liver in lipoprotein metabolism is also key :
- They synthesize VLDL, part of HDL;
- they convert IDL to LDL;
- they degrade chylomicron remnants, HDL and part of LDL.
Protein and amino acid metabolism
- In the metabolism of proteins and amino acids, some reactions are again specific for the liver – urea synthesis . Other reactions, for example the deamination and transamination of amino acids or the synthesis of non-essential amino acids, also take place in other organs. The liver also synthesizes (except immunoglobulins) all plasma proteins, eg albumin or coagulation factors.
See Amino Acid Metabolism for more detailed information .
Kidney
- Concentrating urine and transporting substances in the kidneys requires a large amount of energy, so the consumption of ATP , especially in the cortex, is high.
- ATP is obtained through the oxidative metabolism of glucose, lactate, fatty acids and amino acids.
- From metabolic pathways, gluconeogenesis also takes place here , especially during starvation. Its main substrate is the carbon skeleton of amino acids - mainly glutamine. Ammonia obtained during the reactions is excreted directly into the urine, where it serves as a buffer .
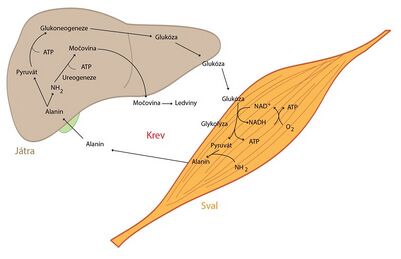
Skeletal muscles
- Skeletal muscles use a large amounts of energy during their activity. ATP regeneration takes place via aerobic and anaerobic glycolysis , fatty acid degradation and also from creatine phosphate .
- The role of skeletal muscle in the metabolism of amino acids , mainly branched (valine, leucine and isoleucine), is essential. Their carbon skeletons are used to generate energy, and their amino groups are used for the synthesis of alanine, glutamine and glutamate, which skeletal muscle releases in large quantities into the circulation. The liver can then regenerate glucose from alanine - the so-called alanine cycle.
See the Glucose-Alanine Cycle page for more detailed information .
Adipose tissue
- Adipose tissue is used postprandially - i.e. after a meal, when the influence of insulin prevails , as a storehouse of triacylglycerols . It stores both dietary lipids and those created by the liver. During fasting, when the effect of glucagon prevails , on the contrary, lipolysis occurs - the release of free fatty acids and glycerol.
Brain
- Glucose is the main energy substrate of the brain, the daily consumption is 120 g. During adapted starvation , which occurs after approximately 3 weeks without an adequate energy supply, the brain can cover up to 50% of the energy consumption by oxidizing ketone bodies .