Keto bodies
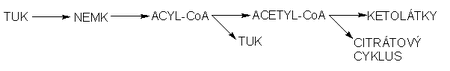
The essence of the formation of ketone bodies is the increased mobilization of fatty acids from adipose tissue and their subsequent transport to the liver . Here, fatty acids are oxidized by the β-oxidation mechanism to acetyl-CoA . Acetyl-CoA molecules are either directly transferred to the citrate cycle , where they are oxidized to carbon dioxide and water to release energy , or they are used for the formation of ketone bodies - acetoacetate, acetone and β-hydroxybutyric acid , which serve as an alternative source of energy in extrahepatic tissues. Ketone substances are produced in increased quantities, especially during starvation or in dietary regimes with strict restriction of carbohydrates or in pathological conditions such as diabetes. Under these conditions, an excess of acetyl-CoA is produced and the capacity of the citrate cycle is increased due to the low concentration of oxaloacetate due to the lack of glucose.
Synthesis of ketone bodies[edit | edit source]
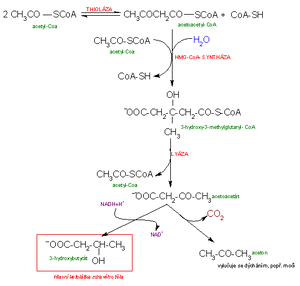
The synthesis of ketone bodies takes place exclusively in the liver in the mitochondrial matrix from acetyl coenzyme A molecules.
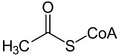
- Acetoacetyl-CoA is an intermediate product of fat breakdown. Acetoacetyl-CoA can be formed by the condensation of two acetyl-CoAs.
- Condensation of acetoacetyl-CoA with an acetyl-CoA molecule results in β-hydroxy-β-methylglutaryl-CoA = HMG-CoA. Under physiological conditions, it is used to create steroid substances such as cholesterol. In plants, it is used for the synthesis of terpenes and carotenes.
- HMG-CoA can be further cleaved by lyase into acetoacetate and acetyl-CoA.
- Z Non-enzymatic (spontaneous) decarboxylation produces acetone from acetoacetate .
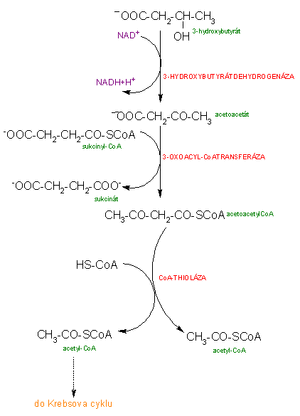
- Another possibility is the reduction of acetoacetate by liver dehydrogenase to β-hydroxybutyric acid (β-hydroxybutyrate).
Conversion of ketone bodies to acetyl-CoA[edit | edit source]
Under normal circumstances, ketone bodies serve as metabolic fuel for some peripheral tissues - heart, skeletal muscle , kidneys , and during prolonged starvation also for brain tissue (60-70%). They are water-soluble equivalents of fatty acids, so their utilization always takes place in the periphery. It does not bind to proteins.
The human body uses only acetoacetate and β-hydroxybutyrate as an energy source. Acetone is exhaled with exhaled air or excreted in the urine. β-Hydroxybutyrate is oxidized to acetoacetate. Acetoacetate must first be activated to the active form acetoacetyl-CoA. The donor of coenzyme A is succinyl-CoA, from which coenzyme A is enzymatically transferred to acetoacetate. The enzyme responsible for this reaction is found in all tissues except the liver, and for this reason ketone bodies are used by extrahepatic tissues, but not by the liver. Acetoacetyl-CoA can be broken down into two acetyl-CoA molecules that are oxidized in the citrate cycle.
Ketosis[edit | edit source]
It occurs when there is a high formation (concentration) of ketone bodies. Acetoacetate passes from the mitochondria into the blood. The metabolism of acetoacetate is slower than its formation.
- Normal ketonemia, level of ketone bodies in the blood: < 0.2 mmol/l [1];
- ketosis - a physiological state during starvation and low-carbohydrate diets, when glycogen is depleted and body fat has become the source of energy, ketonemia 1–3 mmol/l ;
- ketoacidosis - a pathological condition in diabetes, characterized by acidosis, high ketosis > 3 mmol/l , ketonuria, manifested by nausea and vomiting.
Links[edit | edit source]
[edit | edit source]
Sources[edit | edit source]
- ŠKARYDOVÁ, Lucie, Mgr.. Metabolismus tuků a mastných kyselin II. [lecture for subject Obecná biochemie, specialization Farmacie, Farmaceutická fakulta Univerzita Karlova]. Hradec Králové. 29.3.2011.
- STŘEDA, Leoš, Doc. MUDr., Ph.D.. Terapie nadváhy a obezity, aktivní diety [lecture for subject Monitoring a terapie obezity, specialization Lékařství, 1. lékařská fakulta Univerzita Karlova]. Praha. 10.9.2012.
- DOLEČEK, Rajko, Prof. MUDr., DrSc.. Metabolický syndrom [lecture for subject Monitoring a terapie obezity, specialization Lékařství, 1. lékařská fakulta Univerzita Karlova]. Praha. 10.9.2012.
- KOOLMAN, Jan – RÖHM, Klaus-Heinrich. Barevný atlas biochemie. 1. edition. Grada, 2012. 512 pp. ISBN 978-80-247-2977-0.
- MATOUŠ, Bohuslav, et al. Základy lékařské chemie a biochemie. 1. edition. Galén, 2010. 540 pp. ISBN 978-80-7262-702-8.
Reference[edit | edit source]
- ↑ MATOUŠ, Bohuslav – ET AL.,. Základy lékařské chemie a biochemie. 1. edition. Galén, 2010. 540 pp. pp. 156. ISBN 978-80-7262-702-8.