Glycolysis
Glycolysis
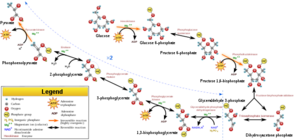
Glycolysis Glycolysis is a metabolic process that converts glucose (or glucose-6-phosphate) into two molecules of pyruvate.
Under anaerobic conditions, pyruvate is converted to lactate catalyzed by lactate dehydrogenase . If the cell has enough oxygen and mitochondria , pyruvate is transferred to the mitochondrial matrix and undergoes oxidative decarboxylation - the reaction of the pyruvate dehydrogenase complex . The regulatory enzymes are 6-phosphofructo-1-kinase, pyruvate kinase (and to some extent hexokinase / glucokinase ).
Glucose phosphorylation
Glucose phosphorylation We must first activate glucose and attach phosphate to it. Glucose-6-phosphate is an important starting point not only for glycolysis but also for the pentose cycle and glycogen synthesis. The reaction is catalyzed by hexokinase (most cells in the body) or glucokinase (hepatocytes, pancreatic β-cells ).
Hexokinase
K m for this enzyme is equal to 0.1 mmol / l, which means that the reaction will proceed at a sufficient rate even at low glucose concentrations. In other words, the enzyme will be almost maximally active at the lower limit of normal blood glucose (3.5 mmol / l).
Glucokinase
The k m for this enzyme is 10 mmol / l, which means that it only works at elevated glucose levels. Its location is of functional significance - β cells thus receive a signal to produce insulin (after a meal, blood glucose increases significantly, so glucokinase begins to work). After raising blood glucose, the liver takes up glucose, which it uses to store glycogen.
Sequence of enzymes and reactions
Glc-6-P isomerization
Glucose-6-phosphate isomerase
This reaction is reversible, giving rise to fructose-6-phosphate . The enzyme acts only on the α-anomer of glucose.
6-Phosphofructo-1-kinase
Phosphorylation of Fru-6-P This reaction is irreversible , the activity of the enzyme can be allosterically affected. It follows that it plays an essential role in the regulation of glycolysis. Mg 2+ acts as a cofactor . The reaction product is fructose-1,6-bisphosphate .
Aldolase
Fru-1,6-BP cleavage, DAP and GAP interconversion Fructose-1,6-bisphosphate undergoes cleavage to two triose phosphates, glyceraldehyde-3-phosphate (GAP) and dihydroxyacetone phosphate (DAP). Since further glycolysis requires GAP, it is necessary to convert DAP to GAP. This is done via the enzyme triose phosphate isomerase .
Glyceraldehyde-3-phosphate dehydrogenase
GAP oxidation This enzyme binds free (inorganic) phosphate (Pi) to GAP (with simultaneous oxidation). Oxidation is an exergonic reaction. The released energy is stored in a macroergic bond with phosphate (simply…), the reaction product is 1,3-bisphosphoglycerate and one molecule of NADH + H +
Phosphoglycerate kinase
The macroergic phosphate formed in the previous reaction is captured in the form of ATP (reaction with ADP). If ATP is formed outside the respiratory chain, we speak of so-called phosphorylation at the substrate level . In addition to ATP, 3-phosphoglycerate is formed in the reaction .
Phosphoglycerate mutase
By this reaction, the phosphate is moved from position 'three' to position 'two'. So we get 2-phosphoglycerate (2-PG).
Enolase
The enolase causes dehydration of 2-PG, and thus the elevation of the phosphate to a high-energy state (or its cleavage, which subsequently occurs, will have serious consequences). Phosphoenolpyruvate is formed . Enolase can be inhibited by fluoride (clinical use: if we want to prevent blood glycolysis before blood glucose determination).
Pyruvate kinase
Pyruvate kinase cleaves phosphate. Thus we get a highly unstable compound - enol-pyruvate . Why is there such instability? Hydrogen bound to oxygen within the hydroxy group tends to move to the carbon, thereby shifting the double bond between the carbon and oxygen, and thus forming a keto group (so-called keto-enol tautomerism). During the rearrangement, energy is released, which is captured in the form of ATP (ADP reacts with the released phosphate). The reaction is irreversible and therefore potentially regulatory. The result is pyruvate (or keto-pyruvate).
Pyruvate transformations
Lactate dehydrogenase
[✎ edit embedded article] Pyruvate is reduced to lactate with NADH consumption
Lactate dehydrogenase ( LD or LDH , EC 1.1.1.27) is an redox enzyme that catalyzes the reversible conversion of lactate to pyruvate . The structure of the molecule consists of 4 subunits with a relative molecular weight of 34,000. Each of these subunits can be either M ( muscle ) or H ( heart ), so there are a total of 5 isoenzymes called LD 1 (with subunit composition H 4 ) to LD 5 (M 4 ). LD is present in the cytoplasm of many tissue cells. It is released into the circulation even with mild tissue damage.
| isoenzyme | subunits | occurrence |
|---|---|---|
| LD 1 | H 4 | myocardium + erythrocytes |
| LD 2 | H 3 M | myocardium + erythrocytes |
| LD 3 | H 2 M 2 | skeletal muscles |
| LD 4 | HM 3 | liver + skeletal muscles |
| LD 5 | M 4 | liver + skeletal muscles |
Examination
An increase in the catalytic concentration of total LD in the serum accompanies a number of diseases. Currently, the determination of total LD activity is used as a non-specific marker of cell lysis, eg in cancer ( leukemia , testicular tumors). A late increase in total LD after myocardial infarction , which can last for up to 15 days, is also characteristic. Due to the high erythrocyte content, haemolysis may falsely increase serum concentrations . The use of LD and its isoenzymes for the diagnosis of acute coronary syndrome is now considered obsolete.
The physiological upper limit of LD for adult men and women is 4.10 µkat / l.
An optical test is used for the determination . The presence of isoenzymes can be determined electrophoretically .
Pyruvate dehydrogenase
[✎ edit embedded article] Pyruvate dehydrogenase The pyruvate dehydrogenase complex is a complex of three enzymes within the mitochondria : pyruvate decarboxylase, dihydrolipoyltransacetylase and dihydrolipoyl dehydrogenase. The complex works as a whole in the presence of coenzymes TPP , NAD + , lipoate in the form of lipoamide, FAD and coenzyme A. Pyruvate dehydrogenase catalyzes the oxidative decarboxylation of pyruvate with acetyl binding to TPP, dihydrolipoyltransacetylase catalyzes the transfer of acetyl from TPP via lipoamide to coenzyme A, and dihydrolipoyl dehydrogenase regenerates lipoamide by FAD, from which FADH 2 is regenerated by NAD +, from which NADH + H + arises . The enzyme is inhibited by arsenic in the oxidation state As (III) (arsenates,…), which blocks the lipoamide.
Energy balance
| Aerobic conditions | --- | Anaerobic conditions | ||
| activation | −2 ATP | --- | activation | -2 ATP |
| directly in glycolysis | 2 x 2 ATP | --- | directly in glycolysis | 2 x 2 ATP |
| reduced coenzymes (shuttles) | 3-5 ATP | --- | reduced coenzymes | consumed for LDH |
| PDH complex | 5 ATP | --- | ||
| oxidation of AcCoA | 20 ATP | --- | ||
| overall | 30-32 ATP | --- | overall | 2 ATP |
Aerobic conditions
When there is enough oxygen in the cell, the respiratory chain consumes reduced coenzymes. The citrate cycle runs at full capacity (supply of reduced coenzymes) and needs acetylcoenzyme A to function. It comes from an irreversible PDH reaction that consumes the pyruvate formed in glycolysis. A small note about shuttles: Reduced coenzymes formed in glycolysis do not diffuse through mitochondrial membranes. Shuttles are used for their transport - Malasataspartate (ultimately the benefit of 5 ATP in the respiratory chain) and Glycerol phosphate ( ultimately the benefit of 3 ATP in the respiratory chain). Therefore, the table under the column "reduced coenzymes (shuttles)" shows 3-5 ATP.
Anaerobic conditions
If the cell does not have mitochondria (erythrocytes, kidney marrow) or enough oxygen (ischemic tissue, skeletal muscle during exercise), the respiratory chain functions limited (or is stopped). At the same time, the events that precede it stagnate (citrate cycle, PDH). LDH therefore converts accumulating pyruvate to lactate. It is gradually excreted in the blood.
When the circulation is disturbed (myocardial infarction, pulmonary embolism, shock), high plasma lactate levels cause a decrease in pH and respiratory acidosis develops .
Anaerobic glycolysis also takes place in the cells of organs that do not have a developed rich vascular supply: lens, cornea .
Regulation
You can find more detailed information on the page Regulation of individual metabolic pathways .
Links
Reference
- ↑ ŠVÍGLEROVÁ, Jitka. Glycolysis [online]. Last revision 18.2.2009, [cited. 2010-12-25]. < http://wiki.lfp-studium.cz/index.php?title=Glykol%C3%BDza&oldid=359 >.
- ↑ Jaroslav Racek et al .: Clinical Biochemistry, second, revised edition, Galén, 2006
References
- DUŠKA, Frantisek. Biochemistry in context, part 1 - basics of energy metabolism. 1st edition. Prague: Karolinum, 2006. ISBN 80-246-1116-3 .
- MURRAY, Robert K .. Harper's biochemistry. 2nd edition. Jinočany: H&H, 1998. ISBN 80-7319-013-3 .