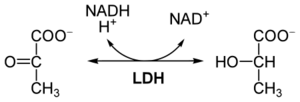
Lactate dehydrogenase
Lactate dehydrogenase ( LD or LDH , EC 1.1.1.27)is an redox enzym that catalyzes the reversible conversion of lactate to pyruvate . The structure of the molecule consists of 4 subunits with a relative molecular weight of 34,000. Each of these subunits can be either M ( muscle ) or H ( heart ), so there are a total of 5 isoenzymes called LD 1 (with subunit composition H 4 ) to LD 5 (M 4 ). LD is present in the cytoplasm of many tissue cells. It is released into the circulation even with mild tissue damage.
| isoenzyme | subunits | occurrence |
|---|---|---|
| LD 1 | H 4 | myocardium + erythrocytes |
| LD 2 | H 3 M | myocardium + erythrocytes |
| LD 3 | H 2 M 2 | skeletal muscles |
| LD 4 | HM 3 | liver + skeletal muscles |
| LD 5 | M 4 | liver + skeletal muscles |
Examination
An increase in the catalytic concentration of total LD in the serum accompanies a number of diseases. Currently, the determination of total LD activity is used as a non-specific marker of cell lysis, eg in cancer ( leukemia , testicular tumors). A late increase in total LD after myocardial infarction , which can last for up to 15 days, is also characteristic. Due to the high erythrocyte content, haemolysis may falsely increase serum concentrations . The use of LD and its isoenzymes for the diagnosis of acute coronary syndrome is now considered obsolete.
The physiological upper limit of LD for adult men and women is 4.10 µkat / l.
An optical test is used for the determination . The presence of isoenzymes can be determined electrophoretically .
Links[edit | edit source]
Related articles[edit | edit source]
Reference[edit | edit source]
Jaroslav Racek et al .: Clinical Biochemistry, second, revised edition, Galén, 2006