Tumors of cerebellum and IV. ventricle in adults
Gliomy[edit | edit source]
Ependymom[edit | edit source]
__
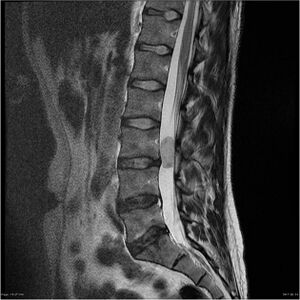
Ependymoma is a neuroepithelial brain tumor, that occurs along the entire length of the nerve axis in the ventricular space. It most often occurs between 1.–5. year of life, most often intracranially (primarily fourth ventricle). [1] The incidence of spinal ependymomas is prevalent in adult patients (most often between 35–45 years of age). They represent the majority (60 %) of all primary intramedullary tumors. [2][3][4][5]
It is benign, semi-malignant, there is also a malignant form. It is well demarcated, may contain focal calcification, may bleed. It can grow anywhere in the ventricular system and spinal cord, grows from the lining of the ventricular system from radial glia. [6][7]
Localization[edit | edit source]
1) 60 % posterior cranial fossa,
2) 30 % supratentorially,
3) 10 % spinal cord. [8]
Diagnostics[edit | edit source]
Ependymomas are primarily diagnosed using CT and MR imaging methods:
- CT – better shows possible calcifications that are present especially in subependymomas,
- MR – ependymomas on MR usually look like well-defined lesions. [9]
Neither edema nor infiltration of the surrounding brain tissue is usually present on both CT and MR. However, as already mentioned in the introduction, in some cases it is possible to see hemorrhage or cystic component. [10]
Biopsy is often performed to properly determine follow-up treatment. [10]
Classification (according to WHO)[edit | edit source]
According to the WHO, we divide ependymomas into three groups [11]:
WHO grade I[edit | edit source]
Division:
- subependymoma – non-invasive, grows slowly, mostly occurs in middle-aged and older patients, often diagnosed incidentally; [12]
- myxopapillary ependymoma – it occurs primarily in the filum terminale and / or in the conus medullaris, represents up to 13% of spinal ependymomas, it is the most common tumor in the cauda equina. [13]
WHO grade II[edit | edit source]
The "classic" ependymoma is the most common of all types of ependymomas. It occurs primarily intracranially. We divide it into:
- papillary ependymoma;
- clear cell ependymoma;
- tanycytic ependymoma – in addition to intracranial areas, it typically occurs in the cervical and thoracic parts of the spinal cord, but it can also occasionally occur in the filum terminale;
- RELA fusion-positive ependymoma (new classification from 2016). [14][15][16][17]
WHO grade III[edit | edit source]
Only one form:
- anaplastic ependymoma – has a higher tendency to proliferate, infiltrates the surrounding brain tissue and metastasizes due to spread by cerebrospinal fluid. [18]
In addition to the WHO classification, we can distinguish these tumors into low-grade a high-grade ependymomas. Low-grade ependymomas are in their biological nature benign, grow slowly (WHO grade I and II). High-grade ependymomas, which include anaplastic ependymoma (WHO grade III), are malignant, grow rapidly and the prognosis of patients with this tumor is not good. [19]
Symptomatology[edit | edit source]
Intracranial ependymomas[edit | edit source]
It primarily depends on the location. In intracranial lesions increased intracranial pressure, headache and related symptoms predominate as the initial presentation. Within the posterior fossa ataxia is common but other symptoms include diplopy, nystagmus etc. Supratentorially, ependymomas are most often manifested by epilepsy, or focal neurological deficits. [20]
Spinal ependymomas[edit | edit source]
Although there is a relatively diverse symptomatology related to individual tumor localization, back pain, sensory deficits and lower limb weakness generally predominate. Some patients may also have bladder dysfunction, ataxic gait, sphincter disorders or sexual problems. Acute exacerbation of symptoms in some patients may be caused by acute intratumoral haemorrhage and occasionally as a result of spinal subarachnoid haemorrhage hydrocephalus may occur. [2][21][22][23][24][25][26][27]
Some ependymomas (most commonly WHO grade II, occasionally WHO grade III) are associated with type 2 neurofibromatosis (NF-2) [28][29][30].
Therapy[edit | edit source]
The basis is the most radical surgical resection. In the case of partial resection, the rest can be irradiated radiosurgically. [31]
Patients (both children and adults) with WHO II or WHO III ependymoma residues are indicated for radiosurgery. It is indicated for chemotherapy, especially in younger children (<12 months) and adult patients with recurrent tumors for whom surgical or radiosurgical treatment is not feasible - it is too risky. [32]
Prognosis[edit | edit source]
It is not very good, especially due to the location of the tumor, which is often difficult to access surgically. The prognosis is primarily affected by:
- localization (the most surgically demanding are those in fourth ventricle),
- anaplastic form of the disease,
- partial resection.
The probability of 5-year survival in children is around 50-70% but in the case of recurrence, mortality is up to 90%. [33]
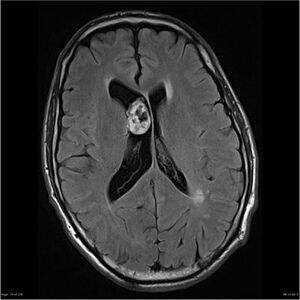
- Ependymoma of IV. brain ventricle (MR)
Diferential diagnostics[edit | edit source]
The following five diseases are often confused with ependymoma, so the basic differences in their diagnosis are presented:
- medulloblastoma – it is very similar to the ependymoma, especially due to the localization (IV. ventricle), it grows out of the vermis, it is not so plastic and calcification is not so frequent; [34]
- papilloma of the chorioid plexus – occurs in children mainly in the trigon of the lateral ventricles and in adults most often in IV. ventricle (this is the opposite typical localization to that of the ependymoma); [35]
- secondary tumor of the chorioid plexus – on MR very similar, but in the case of a secondary tumor of the choroid plexus, the incidence is more common in elderly patients with a history of malignancy; [36]
- Glioblastoma multiforme – is more difficult to distinguish especially from intraparenchymal supratentorial ependymoma, glioblastoma is more common in elderly patients and the epicenter of the tumor is the white matter of the brain; [37]
- central neurocytoma – mostly grows from septum pellucidum. [38]
Links[edit | edit source]
Related articles[edit | edit source]
Used literature[edit | edit source]
- ZEMAN, Miroslav, et al. Speciální chirurgie. 2. edition. Praha : Galén, 2004. 575 pp. ISBN 80-7262-260-9.
- ARNAUTOVIĆ, Kenan – GOKASLAN, Ziya. Spinal Cord Tumors. 1. edition. Springer, 2019. 540 pp. ISBN 9783319994383.
References[edit | edit source]
- ↑ VILLANO, J L – PARKER, C K – DOLECEK, T A. Descriptive epidemiology of ependymal tumours in the United States. British Journal of Cancer. 2013, y. 11, vol. 108, p. 2367-2371, ISSN 0007-0920. DOI: 10.1038/bjc.2013.221.
- ↑ a b CELANO, Emma – SALEHANI, Arsalaan – MALCOLM, James G.. Spinal cord ependymoma: a review of the literature and case series of ten patients. Journal of Neuro-Oncology. 2016, y. 3, vol. 128, p. 377-386, ISSN 0167-594X. DOI: 10.1007/s11060-016-2135-8.
- ↑ CHAMBERLAIN, Marc C.. Ependymomas. Current Neurology and Neuroscience Reports. 2003, y. 3, vol. 3, p. 193-199, ISSN 1528-4042. DOI: 10.1007/s11910-003-0078-x.
- ↑ VILLANO, J L – PARKER, C K – DOLECEK,. Descriptive epidemiology of ependymal tumours in the United States. British Journal of Cancer. 2013, y. 11, vol. 108, p. 2367-2371, ISSN 0007-0920. DOI: 10.1038/bjc.2013.221.
- ↑ MALDJIAN, Joseph A. – PATEL, Rita S.. Cerebral neoplasms in adults. Seminars in Roentgenology. 1999, y. 2, vol. 34, p. 102-122, ISSN 0037-198X. DOI: 10.1016/s0037-198x(99)80025-x.
- ↑ POPPLETON, H – GILBERTSON, R J. Stem cells of ependymoma. British Journal of Cancer. 2006, y. 1, vol. 96, p. 6-10, ISSN 0007-0920. DOI: 10.1038/sj.bjc.6603519.
- ↑ TAYLOR, Michael D. – POPPLETON, Helen – FULLER, Christine. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005, y. 4, vol. 8, p. 323-335, ISSN 1535-6108. DOI: 10.1016/j.ccr.2005.09.001.
- ↑ MALDJIAN, Joseph A. – PATEL, Rita S.. Cerebral neoplasms in adults. Seminars in Roentgenology. 1999, y. 2, vol. 34, p. 102-122, ISSN 0037-198X. DOI: 10.1016/s0037-198x(99)80025-x.
- ↑ CHAMBERLAIN, Marc C.. Ependymomas. Current Neurology and Neuroscience Reports. 2003, y. 3, vol. 3, p. 193-199, ISSN 1528-4042. DOI: 10.1007/s11910-003-0078-x.
- ↑ a b RENI, Michele – GATTA, Gemma – MAZZA, Elena. Ependymoma. Critical Reviews in Oncology/Hematology. 2007, y. 1, vol. 63, p. 81-89, ISSN 1040-8428. DOI: 10.1016/j.critrevonc.2007.03.004.
- ↑ CANCER, International. WHO Classification of Tumours of the Central Nervous System. - edition. 2007. 309 pp. ISBN 9789283224303.
- ↑ VARMA, Adithya – GIRALDI, David – MILLS, Samantha. Surgical management and long-term outcome of intracranial subependymoma. Acta Neurochirurgica. 2018, y. 9, vol. 160, p. 1793-1799, ISSN 0001-6268. DOI: 10.1007/s00701-018-3570-4.
- ↑ FRAZIER, Aletta Ann. Myxopapillary Ependymoma. RadioGraphics. 2019, y. 2, vol. 39, p. 467-467, ISSN 0271-5333. DOI: 10.1148/rg.2019184014.
- ↑ SCHELLINGER, Kate A. – PROPP, Jennifer M. – VILLANO, J. Lee. Descriptive epidemiology of primary spinal cord tumors. Journal of Neuro-Oncology. 2007, y. 2, vol. 87, p. 173-179, ISSN 0167-594X. DOI: 10.1007/s11060-007-9507-z.
- ↑ BOCCARDO, M – TELERA, S – VITALI, A. Tanycytic ependymoma of the spinal cord. Case report and review of the literature. Neurochirurgie [online]. 2003, vol. 49, p. 605-10, Available from <https://www.ncbi.nlm.nih.gov/pubmed/14735006>. ISSN 0028-3770.
- ↑ HOU, Zonggang – TAO, Xiaogang – ZHANG, Junting. Tanycytic ependymoma of filum terminale: Clinical characteristics and surgical outcomes. Oncology Letters. 2018, y. ?, vol. ?, p. ?, ISSN 1792-1074. DOI: 10.3892/ol.2018.9531.
- ↑ FUKUSHIMA, Tsuyoshi – UEDA, Takashi – HIRATO, Junko. RELA fusion-positive ependymoma accompanied by extensive desmoplasia: a case report. Brain Tumor Pathology. 2020, y. 4, vol. 37, p. 159-164, ISSN 1433-7398. DOI: 10.1007/s10014-020-00376-w.
- ↑ NEUMANN, Julia E. – SPOHN, Michael – OBRECHT, Denise. Molecular characterization of histopathological ependymoma variants. Acta Neuropathologica. 2019, y. 2, vol. 139, p. 305-318, ISSN 0001-6322. DOI: 10.1007/s00401-019-02090-0.
- ↑ HÜBNER, Jens-Martin – KOOL, Marcel – PFISTER, Stefan M. Epidemiology, molecular classification and WHO grading of ependymoma. J Neurosurg Sci [online]. 2018, vol. 62, p. 46-50, Available from <https://doi.org/10.23736/S0390-5616.17.04152-2>. ISSN 0390-5616 (print), 1827-1855.
- ↑ PRINCE, M R – CHEW, F S. Ependymoma of the fourth ventricle.. American Journal of Roentgenology. 1991, y. 6, vol. 157, p. 1278-1278, ISSN 0361-803X. DOI: 10.2214/ajr.157.6.1950882.
- ↑ PAJTLER, Kristian – GERSTNER, Elizabeth. Ependymoma. Seminars in Neurology. 2018, y. 01, vol. 38, p. 104-111, ISSN 0271-8235. DOI: 10.1055/s-0038-1636503.
- ↑ BAGLEY, Carlos A. – WILSON, Sean – KOTHBAUER, Karl F.. Long term outcomes following surgical resection of myxopapillary ependymomas. Neurosurgical Review. 2009, y. 3, vol. 32, p. 321-334, ISSN 0344-5607. DOI: 10.1007/s10143-009-0190-8.
- ↑ BALASUBRAMANIAM, Srikant – TYAGI, Devendra K – DESAI, Ketan I. Outcome Analysis in Cases of Spinal Conus Cauda Ependymoma. J Clin Diagn Res [online]. 2016, vol. 10, p. PC12-PC16, Available from <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5072009/?tool=pubmed>. ISSN 0973-709X (print), 2249-782X.
- ↑ – VANLANDINGHAM, Matthew – O'BRIEN, Thomas. Primary Seeding of Myxopapillary Ependymoma: Different Disease in Adult Population? Case Report and Review of Literature. World Neurosurgery. 2017, y. ?, vol. 99, p. 812.e21-812.e26, ISSN 1878-8750. DOI: 10.1016/j.wneu.2016.12.022.
- ↑ MORIMOTO, Daijiro – ISU, Toyohiko – KIM, Kyongsong. Surgical treatment for posttraumatic hemorrhage inside a filum terminale myxopapillary ependymoma: a case report and literature review. European Spine Journal. 2016, y. S1, vol. 25, p. 239-244, ISSN 0940-6719. DOI: 10.1007/s00586-016-4521-5.
- ↑ TERAO, Tohru – KATO, Naoki – ISHII, Takuya. Spontaneous Hemorrhage of a Spinal Ependymoma in the Filum Terminale Presenting with Acute Cauda Equina Syndrome: Case Report. NMC Case Report Journal. 2016, y. 3, vol. 3, p. 91-95, ISSN 2188-4226. DOI: 10.2176/nmccrj.cr.2015-0295.
- ↑ RIVIEREZ, M. – OUESLATI, S. – PHILIPPON, J.. [Ependymoma of the intradural filum terminale in adults. 20 cases]. Neurochirurgie [online]. 1990, vol. 36, no. 2, p. 96-107, Available from <https://www.ncbi.nlm.nih.gov/pubmed/2164165>. ISSN 0028-3770.
- ↑ ARNAUTOVIĆ, Kenan – GOKASLAN, Ziya. Spinal Cord Tumors. 1. edition. Springer, 2019. vol. 540. ISBN 9783319994383.
- ↑ ARDERN-HOLMES, Simone – FISHER, Gemma – NORTH, Kathryn. Neurofibromatosis Type 2. Journal of Child Neurology. 2016, y. 1, vol. 32, p. 9-22, ISSN 0883-0738. DOI: 10.1177/0883073816666736.
- ↑ TAO, Xiao-Gang – HOU, Zong-Gang – HAO, Shu-Yu. Two Cases of Spinal Tanycytic Ependymoma Associated with Neurofibromatosis Type 2. Chinese Medical Journal. 2017, y. 7, vol. 130, p. 872-873, ISSN 0366-6999. DOI: 10.4103/0366-6999.202732.
- ↑ PAJTLER, Kristian W. – MACK, Stephen C. – RAMASWAMY, Vijay. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathologica. 2016, y. 1, vol. 133, p. 5-12, ISSN 0001-6322. DOI: 10.1007/s00401-016-1643-0.
- ↑ RUDÀ, Roberta – REIFENBERGER, Guido – FRAPPAZ, Didier. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro-Oncology. 2017, y. 4, vol. 20, p. 445-456, ISSN 1522-8517. DOI: 10.1093/neuonc/nox166.
- ↑ SMITH, Alice Boyd – SMIRNIOTOPOULOS, James G. – HORKANYNE-SZAKALY, Iren. From the Radiologic Pathology Archives: Intraventricular Neoplasms: Radiologic-Pathologic Correlation. RadioGraphics. 2013, y. 1, p. 21-43, ISSN 0271-5333. DOI: 10.1148/rg.331125192.
- ↑ BEHARI, Sanjay – JAISWAL, AwadheshKumar – JAISWAL, Sushila. Choroid plexus papilloma in children: Diagnostic and surgical considerations. Journal of Pediatric Neurosciences. 2009, y. 1, vol. 4, p. 10, ISSN 1817-1745. DOI: 10.4103/1817-1745.49100.
- ↑ PARK, Sung Hye – PARK, Heum Rye – CHI, Je G. Papillary ependymoma: its differential diagnosis from choroid plexus papilloma. Journal of Korean Medical Science. 1996, y. 5, vol. 11, p. 415, ISSN 1011-8934. DOI: 10.3346/jkms.1996.11.5.415.
- ↑ ESCOTT, Edward J.. A Variety of Appearances of Malignant Melanoma in the Head: A Review. RadioGraphics. 2001, y. 3, vol. 21, p. 625-639, ISSN 0271-5333. DOI: 10.1148/radiographics.21.3.g01ma19625.
- ↑ LOUIS, David N. – PERRY, Arie – REIFENBERGER, Guido. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica. 2016, y. 6, vol. 131, p. 803-820, ISSN 0001-6322. DOI: 10.1007/s00401-016-1545-1.
- ↑ KOELLER, Kelly K. – SANDBERG, Glenn D.. From the Archives of the AFIP. RadioGraphics. 2002, y. 6, vol. 22, p. 1473-1505, ISSN 0271-5333. DOI: 10.1148/rg.226025118.
</noinclude>
Hemangioblastom[edit | edit source]
- dříve nazývaný angioretikulom
- v 80 % je to spontánně se vyskytující tumor
- z 20 % je součástí geneticky podmíněného syndromu – syndrom von Hippel-Lindau (VHL) (hemangiomy CNS, angiomatóza sítnice, cysty parenchymatózních orgánů)
- u 25 % z nich se vyskytují karcinom ledvin, feochromocytom a častěji též karcinom pankreatu!
- je benigní, postihuje hlavně mladší jedince
- nejčastěji je v mozečku
- je často cystický, nebo může být solidní, silně vaskularizovaný
- právě cystická forma je nebezpečná, protože se může začít rychle zvětšovat a ohrožuje na životě akutní okcipitální herniací!
- klinický obraz – mozečkový syndrom a syndrom nitrolební hypertenze
- u 20 % je polyglobulie z ektopické produkce EPO nádorem
- diagnóza – nejlépe na MRI s Gd kontrastem – cysta s hyperdenzním nádorovým uzlíkem
- terapie – cystický hemangioblastom je vždy indikací k urgentní operaci!, kdykoli může dojít ke konu a během minut ke smrti, nutné pátrat v rodině po VHL nemoci, hledat sonem cysty na orgánech, …
Links[edit | edit source]
Source[edit | edit source]
- BENEŠ, Jiří. Studijní materiály [online]. ©2007. [cit. 2009]. <http://www.jirben.wz.cz/>.
Použitá literatura[edit | edit source]
- ZEMAN, Miroslav. Speciální chirurgie. 2. edition. Praha : Galén, 2004. 575 pp. ISBN 80-7262-260-9.