Blood
Composition of Blood[edit | edit source]
Blood is a specialized connective tissue that is composed of:
- Fluid =plasma
- Cells = formed elements of blood:
- Red blood cells (RBC)
- White blood cells (WBC)
- Platelets (PLT)
There is about 5L of blood in an average adult of 60Kg body weight. It is circulating unidirectional within a closed circulatory system with a central pump, the heart.
A tube of blood after centrifugation (center) has:
- Hematocrit: is a volume about 43% of its volume represented by erythrocytes in the bottom half of the tube
- Buffy coat: Is a volume about 1% between the sedimented erythrocytes and the supernatant light—colored plasma. It’s a thin layer of leukocytes and platelets. A cubic millimeter (mm3) of blood is equivalent to a microliter (µL)
- Plasma is the supernatant light colored and contains clotting factors + protein + water
Comparison of Plasma and Serum[edit | edit source]
Blood can be collected in a tube with anticoagulant (heparin or citrate) and centrifuged, so as to be separated in:
- Plasma ( proteins + clotting factors + water)
- Buffy coat ( platelets and WBC) = 1%
- Red blood cells ( that represent the hematocrit= the percentage % volume of the RBC in comparison with the total blood = 43-45%
Blood collected in plane tube without anticoagulant and centrifuged can be separated in:
- Serum ( proteins and water without clotting factors that are consumed in the process of coagulation)
- Layer of coagulation ( fibrin + platelets + WBC)
- Red blood cells
Serum is the liquid part after the coagulation. Serum contains proteins, albumin and globulins (a1, a2, b1, b2 and gamma globulins). All these proteins are possible to be separated with a process named protein electrophoresis.
Plasma and serum are two very common terms we hear on a regular basis. What is difference between the two? The main difference between plasma and serum lies in their clotting factors. Plasma contains all clotting factors while serum is produced after we let the blood to coagulate, so it’s free of clotting factors. What remains of the blood once the red blood cells, white blood cells and clotting factors have been removed? Blood serum is mostly water that is dissolved with proteins, hormones, minerals and carbon dioxide.
SUMMARY:
- Plasma is the part of the blood that contains Serum+ clotting factors
- Serum is the part of the blood that remains once the clotting factors like fibrin have been removed
- Plasma contains clotting factors and water , while serum contains proteins like albumin and globulins
Medical Applications[edit | edit source]
Blood Donation[edit | edit source]
A blood donation is taken by an aseptic technique into plastic bags containing an appropriate amount of anticoagulant-usually CPD (citrate, phosphate, dextrose). The citrate, by combining with the blood calcium acts as anticoagulant. Three components are made by initial centrifugation of whole blood: red cells, buffy coat and plasma.
1. The red blood cells (The packed red blood cells=plasma depleted):
- Are stored at 4-6 oC for up to 35 days depending on the preservative
- The packed red blood cells are the treatment of choice for most transfusions
- Are used in patients with hematological diseases as Thalassemia (congenital anemia), and in other acquired anemia (blood loss, operations, accidents with bleeding)
2. The plasma:
- The plasma is derived after the whole blood is treated with anticoagulants. This plasma is possible to be stored frozen for a long time even years after the date has been collected. Usually is given in patients with problems in blood clotting. It is used in bleeding disorders when we don’t know exactly the cause of bleeding and the exactly deficient factor.
- From the plasma we can separate the different coagulation factors. For example it’s possible to be produce fibrinogen, Factor VII, factor VIII, FactorIX, and XI (the last three used in Hemophilia)
- From fresh frozen plasma with further fractionation we can separate albumins, gamma globulins and specific antiviral immunoglobulins,
3. The platelets
- The platelets are used in condition of thrombocytopenia (low platelet count) or in condition of dysfunction of platelet.
- Platelet transfusions can be used for prophylaxis of bleedings (platelet has to be kept >50.000/ml) or for treatment of bleedings (when the platelet number is very low <20.000/ml and there is grade possibility of bleedings).
Erythrocytes[edit | edit source]
Red blood cells or erythrocytes took their name from the Greek word “ερυθρό” = erythro = red color. The RBC is unique amongst eukaryotic cell that:
- it is anuclear (without nucleus),
- It has no cytoplasmic structures/ organelles.
- Its structural properties are linked to the membrane.
- 7.5 micron cell needs to be able to deform to pass through 3 micron capillaries/ reticulo-endothelial system without fragment
The normal concentration of erythrocytes in blood is in function of age and sex. In average is approximately:
- -3.9-5.5 million /ml in women and
- -4.1-6.0 million/ml in men
Erythrocytes are terminally differentiated cells, they don’t have nuclei but they contain a specific protein, the hemoglobin, which carries the oxygen in blood. Under normal conditions the red blood cells never leave the circulatory system. Normally human RBC survive in circulation for about 120 days.
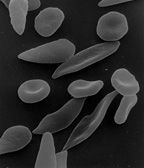
Erythrocytes are flexible biconcave disks with diameter approximately 7.5 μm. This biconcave shape provides a large surface-to-volume ratio and facilitates gas exchange. Due to the fact that the erythrocytes are flexible help them to adapt to the irregular bends and small diameter of capillary. In vivo observations show that the normal erythrocytes with normal hemoglobin (HbA) are easily deformed and due to their flexibility they can traverse the angles and the bifurcation of the capillary vessels. In contrary, pathological red blood cells, like sickle cell erythrocytes, where the hemoglobin is HbS, are misshapen cells, with reduced flexibility.
Medical Applications[edit | edit source]
Sickle cell[edit | edit source]
The sickle cells are pathological red blood cells, the hemoglobin is HbS, are misshapen cells, with reduced flexibility. The problem, however, is not simply one of abnormal shape. The membranes of the cells are rigid due in part to repeat episodes of hemoglobin polymerization/depolymerization as the cells pick up and release oxygen in the circulation. These rigid cells fail to move through the small blood vessels, blocking local blood flow to a microscopic region of tissue. Amplified many times, these episodes produce tissue hypoxia (low oxygen supply). The result is pain, (painful vaso-occlusive crises) and often damage to organs.
Normal red cells maintain their biconcave shape as they pass through the capillaries and release oxygen to the peripheral tissues (upper panel). In contrast, hemoglobin polymers form in the sickle red cells with oxygen release, causing them to deform. The deformed cells block the flow of cells and interrupt the delivery of oxygen to the tissues.
Molecular Pathology of Sickle cell anemia:
| Header text | Header text | Header text | Header text | Header text |
|---|---|---|---|---|
| Amino Acids | PRO | GLU | GLU | |
| Normal β Chain | ||||
| Base Composition | CCT | GAG | GAG | |
| Base Composition | CCT | GTG | GAG | |
| Sickle β Chain | ||||
| Amino Acids | PRO | VAL | GLU |
There is a single base change in DNA coding for the amino acid in the 6th position in the b-globin chain: Adenine is replaced by Thymine. This leads to an amino acid change From Glutamic acid to Valine
A: Adenine, G: Guanine, C: Cytosine, T: Thymine
GLU: Glutamic acid, PRO: Proline, VAL: Valine
Structure of Erythrocyte membrane[edit | edit source]
The membrane of erythrocyte consists of:
- 40% lipid ( double layer of phospholipids and cholesterol)
- 10% carbohydrates
- 50% protein.
•Membrane Proteins includes:
- Integral proteins: (are directly incorporated within lipid bilayer)
- -as ion channels K+, Na+, Ca+2
- Integral Transmembrane proteins (are large enough to extend across the two lipid layers) or Anion transporter called :
- -Band 3 protein. Exchanges bicarbonate for chloride
- -Glycophorin A. The glycosylated extracellular domain of these proteins includes antigenic sites that form the basis of blood typing.
- Peripheral proteins : (exhibit a looser association with one of the membrane surfaces) are responsible for the shape and the flexibility of the red blood cells required for normal passage through the capillaries and important for the blood viscosity.These are:
- Spectrin
- forms the red cell membrane skeleton.
- Is responsible for biconcave shape of the RBC
- The basic unit is a hexagonal lattice with 6 spectrin molecules.
- Has two subunits alpha and beta, entwined to form dimers.
- Associate head to head to form tetramers
- Spectrin Mutations lead to congenital elliptocytosis (disturbance of the shape of the RBC that are “ελλειπτοκύτταρα”=they have shape as a thin cylinder)
- Actin
- Short, uniform filaments 35nm in length.
- Length modulated by tropomyosin/ tropomodulin.
- Spectrin tail associated with actin filaments.
- Approximately 6 spectrin ends interface with one actin filament, stabilized by protein 4.1
- Ankyrine
- links the lipid bilayer to membrane skeleton via interaction with band 3.
Structure of RBC membrane:
Hemoglobin[edit | edit source]
- Erythrocyte cytoplasm is densely filled with hemoglobin
- The main function of hemoglobin (Hb) is to carry O2 to the tissues and to return carbon dioxide (CO2) from the tissues to the lungs
- In adult blood the dominant Hb is Hemoglobin A and small quantities of HbA2 and Hb F
Normal Hemoglobin in adult blood
| Header text | Header text | Header text | Header text |
|---|---|---|---|
| HbA | HbF | HbA2 | |
| Structure | α2β2 | α2γ2 | α2δ2 |
| Normal % | 96-98 | 0.5-0.8 | 1.5-3.2 |
Each molecule of normal HbA consists of four polypeptide chains α2β2, each with its own haem group.
These haem groups contain positively-charged iron (Fe2+) molecules which can reversibly bind to oxygen molecules and transport them to various areas of the body. As the haem groups bind or release their oxygen loads, the overall hemoglobin undergoes conformational changes which alters their affinity for oxygen. When hemoglobin is combined with oxygen it forms oxyhemoglobin. When it is combined with CO2 it forms carbaminohemoglobin or deoxyhemoglobin. These combinations are reversible and this is the basis of the gas transporting capability of hemoglobin. The combination of hemoglobin with carbon monoxide is irreversible and thus reduces the cell’s capacity to transport O2.
The amount of O2 in blood (the O2 pressure) is highest in arteries and lung capillaries and decreases in tissue capillaries, where exchange takes place between blood and tissues.
Medical Applications[edit | edit source]
Anaemia[edit | edit source]
Anaemia is the decreased of hemoglobin under the normal limits for age and sex (adult women <12g/dl, adult man <13/g/dl). Although anemias are usually associated with decreased number of erythrocytes, it is also possible for the number of cells to be normal or even increased, but each cell to contain a reduced amount of hemoglobin
I) Microcytic anemia: The red blood cells are very large with diameter <7μm
- 1) In iron deficiency after blood loos or iron deficiency in the diet. The red blood cells count usually is normal but the RBC are very small (with diameter <6μ) and hypochromic (very pale with a white ring in the center)
- 2) In Thalassemia major or minor due to genetic defect of α or β polypeptide chains of hemoglobin. In this condition, the red blood cell count is increased but the RBC are very small, hypochromic and with different sizes and shapes (anisopoikilocytosis)
The RBC's here are smaller than normal and have an increased zone of central pallor. This is indicative of a hypochromic (less hemoglobin in each RBC) microcytic (smaller size of each RBC) anemia. There is also increased anisocytosis (variation in size) and poikilocytosis (variation in shape).
II)Macrocytic anemia: The red blood cells are very large with diameter > 9μm
1)In Vitamin B12 deficiency due to
- a. Dietary habits for ex. In vegetarian (vitamin B12 is produced by bacteria found in dirt that cling to plant roots[1], can be found in water[2], but has strongest concentrations in meat).
- b. Disturbance in absorption of Vitamin B12 ex. In gastric atrophy due to the deficiency of Intrinsic Factor that is synthetized by parietal gastric cells. This factor is indispensable, for Vitamin B12 absorption.
- c. After gastrectomy
2)In Folate deficiency
- a. Poor diet
- b. Increased utilization for ex.in pregnancy and lactation
- c. Malabsorption due to intestine inflammatory diseases as Crohn’s disease
Anaemia is possible to be produced also due to:
- insufficient production of erythrocytes by the bone marrow in case of bone marrow failure due to leukemia or lymphomas
- increased destruction, of erythrocytes due to the autoimmune diseases (haemolytic anaemia)
Polycythaemia[edit | edit source]
Polycythemia is the increase of hemoglobin or hematocrit above the upper limit of normal for age and sex (adults woman Hb> 16g/dl, Htc>47% and man Hb> 17g/dl, Htc>50%). It can occur as a primary phenomenon, i.e. as a stem cell disorder when we have a clonal malignancy of a marrow stem cell. This disease results from a somatic mutation of a single haemopoietic stem cell which gives its progeny a proliferation advantage. It can also occur secondary as a physiological adaptation in individuals who live in high altitudes where the (O2) oxygen tension is low.
Links[edit | edit source]
Related Articles[edit | edit source]
Bibliography[edit | edit source]
- MESCHER, Antony L.. Junqueira's Basic Histology. 12th Editions edition. 2010. ISBN 978-0-07-163020-7.
- HOFFBRAND, A.V. Essential Haematology. 5th Edition edition. 2008. ISBN 978-1-4051-8956-9.
- ΛΟΥΚΌΠΟΥΛΟΣ, Δημήτρης. Αιματολογία με μια ματιά. 1st Edition edition. 2000. ISBN 0-632-04793-3.
References[edit | edit source]
- http://www.google.com.cy/imgres?q=oxyhaemoglobin&hl=en&sa=X&biw=1280&bih=909&tbm=isch&prmd=imvns&tbnid=cQejTpRFOuF97M:&imgrefurl=http://www.experimentalphysiology.
- http://www.ncbi.nlm.nih.gov/books/NBK9898/
- http://sickle.bwh.harvard.edu/scd_background.html
- http://www.acbd.monash.org/docs/red-cell-membrane.pdf
- http://library.med.utah.edu/WebPath/HEMEHTML/HEME008.html
- http://sickle.bwh.harvard.edu/scd_background.html