Amylase
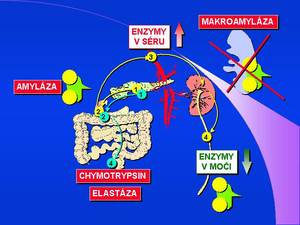
α-amylase[1] [2][3][4][5][6][7][8][9][10](AMS, α-1,4-glucan-4-glucan hydrolase, EC 3.2.1.1) hydrolyses the α-1-4-glycoside bond; The pH optimum of α-amylase is between 7.0-7.2. It occurs in the body in two forms - as a salivary and pancreatic isoenzyme according to organ origin. Both isoforms of AMS differ from each other in the sugar component and can be distinguished electrophoretically or by precipitation using a special lectin or antibody[11]. α-amylase is formed in the acinar cells of the pancreas and accumulates in zymogenic granules. It enters the intestinal lumen in the form of pancreatic secretion (pancreatic juice) along with other digestive enzymes. Under physiological conditions, the enzyme molecule is not absorbed by the intestinal surface and the serum level is low, corresponding to the activity of the enzyme released into the circulation directly from the glandular cells, resp. lymphatic drainage. The molecular weight of α-amylase is 55,000. Α-Amylase is eliminated from the circulation in the kidneys by glomerular filtration. The macroform of the enzyme (macroamylase)[12][13][14][15][16] is formed by the binding of the enzyme to certain proteins in the blood serum, especially immunoglobulins, circulating immunocomplexes or other glycoproteins. The macroform of the enzyme has a significantly higher molecular weight (from 150,000 to 2,000,000) and is therefore not eliminated by glomerular filtration. For clinical diagnosis, serum and urinary α-amylase levels are determined and the amylase / creatinine clearance index is calculated.
Laboratory determination of α-amylase concentration
In the laboratory, protein concentration can be determined by immunological techniques or enzyme catalytic concentration using specific substrates. The presence of inhibitors in the serum and the formation of enzyme macroforms should be considered when determining both mass and catalytic enzyme concentrations. The commonly used determination of α-amylase activity is based on the cleavage of a chromogenic substrate. Older processes that used derivatives of the natural substrate, starch, were difficult to standardize and are no longer used. Current synthetic substrates are derived from maltose, as chromogen is the most commonly used 4-nitrophenyl phosphate. The determination of α-amylase isoenzymes is made possible by the inhibition of one of the two isoenzymes by a specific monoclonal antibody.
Links
Related articles
Source
- https://www.wikiskripta.eu/w/Amyláza/stanoven%C3%AD
- accepted with the permission of the author from KOCNA, Petr. GastroLab : MiniEncyklopedie laboratorních metod v gastroenterologii [online]. ©2002. Poslední revize 2011-01-08, [cit. 2011-03-04].
Total α-amylase in diagnosis
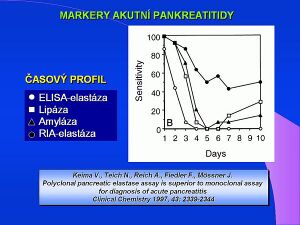
Elevated levels of total α-amylase activity are evident in a number of diseases. The most common diagnostic reason is suspected acute pancreatitis. Although the level of total α-amylase is increased in 100% of acute pancreatitis, it is also increased in 80% of all cases of acute abdominal pain. The determination of the pancreatic isoenzyme α-amylase (β-amylase) has a significantly greater diagnostic benefit, the level of which is also increased in 100% of cases of acute pancreatitis, and is only increased in 10% of acute abdominal pain. In routine clinical practice, the criterion of a five-fold increase in total amylase is used, which is a diagnostic indicator of acute pancreatitis. Elevated serum levels are, of course, demonstrable in both acute and chronic kidney diseases, in intestinal inflammations in 30% of cases of acute appendicitis. The increase in urinary α-amylase levels in acute pancreatitis persists longer and occurs later than the increase in serum levels. The salivary type of α-amylase (S-amylase) is increased in diseases of the salivary glands, in some lung diseases, in a number of malignant tumors, ovarian cysts, ectopic pregnancy. Normal values of the ratio of amylase to creatinine clearance are between 2-4%, in pancreatitis the index rises to 10%. An increased amylase / creatinine clearance index has been demonstrated, for example, in diabetic ketoacidosis, burns, myeloma and renal disorders. Decreased clearance index is diagnostically significant for macroamylasemia.
There are also specific methods for the determination of salivary α-amylase, such as monoclonal antibody ELISA.
Detection of macroamylase complex in serum
Macroamylase and is the cause of 8–12% of cases of hyperamylase (increased values of total serum α-amylase). The cause is a macrocomplex of the enzyme with high molecular weight serum proteins, especially with immunoglobulins IgA and IgG. A similar macrocomplex is formed by other enzymes, such as pancreatic lipase. The α-amylase macroform can be determined by gel filtration, electrophoresis, PEG precipitation or ELISA. Quantitative determination of the enzyme macroform ratio is most accurate using gel permeation chromatography on Sephadex® G-100. The separation takes place in a refrigerated box at 10 ° C on a 9 × 150 mm column at a flow rate of 3 ml / hour for 5 hours. The α-amylase activity in the individual fractions is determined enzymatically by a standard procedure with pNP blocked maltoheptaoside and the result is the ratio of both forms of the enzyme.
An important aspect for the detection of the macroamylase complex is the instability of the complex with immunoglobulins. The complexes dissociate during serum freezing and thawing, so macroamylase must be detected in a freshly collected serum sample.
Reference values: The macroform of the enzyme is not present under physiological conditions. The test result is given as a percentage of the amylase macroform calculated from the activities of the individual fractions. A positive result, a proof of the complex, is therefore a value> 0.
Kategorie:Vložené články Kategorie:Biochemie Kategorie:Klinická biochemie Kategorie:Gastroenterologie
Determination of pancreatic amylase in stool
Determination of pancreatic amylase in faeces [17][18][19][20] is a relatively little used test, which is also an immunochemical test with an antibody to human pancreatic amylase - a novelty that appeared in 2000. Normal values are above 360 µg / g stool, calibration standards are in the range of 0-12,000 µg / g. The test is based on the ILMA (ImmunoLuminoMetric Assay) principle with a luminescent measurement marker being acridinium ester. The original work on the determination of pancreatic amylase in stool mainly describes the detection of isoenzymes and the correlation with other pancreatic enzymes in stool in patients with pancreatic insufficiency.
Links
Related articles
Source
- https://www.wikiskripta.eu/w/Amyláza/stanoven%C3%AD_ve_stolici
- With the permission of the author taken from KOCNA, Petr. GastroLab: MiniEncyclopedia of laboratory methods in gastroenterology [online]. © 2002. Last revision 2011-01-08, [cit. 2011-03-04]. < http://www1.lf1.cuni.cz/~kocna/glab/glency1.htm >.
References
- ↑ CARROLL, Jennifer K, Brian HERRICK a Teresa GIPSON. Acute Pancreatitis: Diagnosis, Prognosis, and Treatment. American Family Physician [online]. 2007, vol. 75, no. 10, s. 1513-1520, dostupné také z <https://www.aafp.org/afp/2007/0515/p1513.pdf>. ISSN 0002-838X.
- ↑ KOCNA, Petr a Tomáš ZIMA. Hyperamylazémie, laboratorní a klinické aspekty. Časopis lékařů českých [online]. 2006, vol. 145, s. 449-452, dostupné také z <https://www.prolekare.cz/casopis-lekaru-ceskych-clanek?id=3093>. ISSN 1803-6597.
- ↑ QUARINO, L, Q DANG a J HARTMANN. An ELISA method for the identification of salivary amylase. Journal of forensic sciences. 2005, vol. 50, no. 4, s. 873-876, ISSN 0022-1198.
- ↑ SMITH, Ross C, James SOUTHWELL-KEELY a Douglas CHESHER. Should serum pancreatic lipase replace serum amylase as a biomarker of acute pancreatitis?. ANZ journal of surgery [online]. 2005, vol. 75, no. 6, s. 399-404, dostupné také z <https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1445-2197.2005.03391.x?cookieSet=1>. ISSN 1445-1433.
- ↑ PANTEGHINI, Mauro, Ferruccio CERIOTTI a Franca PAGANI, et al. Recommendations for the routine use of pancreatic amylase measurement instead of total amylase for the diagnosis and monitoring of pancreatic pathology. Clinical Chemistry and Laboratory Medicine [online]. 2002, vol. 40, no. 2, s. 97-100, dostupné také z <https://www.degruyter.com/doi/abs/10.1515/CCLM.2002.017>. ISSN 1434-6621.
- ↑ TREACY, John, Anthony WILLIAMS a Renz BAIS, et al. Evaluation of amylase and lipase in the diagnosis of acute pancreatitis. ANZ Journal of Surgery [online]. 2001, vol. 71, no. 10, s. 577-82, dostupné také z <https://onlinelibrary.wiley.com/doi/pdf/10.1046/j.1445-2197.2001.02220.x?cookieSet=1>. ISSN 1445-1433.
- ↑ PEZZILLI, R, G TALAMINIL a L GULLO. Behaviour of serum pancreatic enzymes in chronic pancreatitis. Digestive and Liver Disease. 2000, vol. 32, no. 3, s. 233-237, ISSN 1590-8658.
- ↑ KEIM, V, N TEICH a F FIEDLER, et al. A comparison of lipase and amylase in the diagnosis of acute pancreatitis in patients with abdominal pain. Pancreas. 1998, vol. 16, no. 1, s. 45-49, ISSN 0885-3177.
- ↑ CHASE, CW, DE BARKER a WL RUSSELL. Serum amylase and lipase in the evaluation of acute abdominal pain. The American surgeon. 1996, vol. 62, no. 12, s. 1028-1033, ISSN 0003-1348.
- ↑ MALFERTHEINER, P a J ENRIQUE DOMÍNGUEZ-MUÑOZ. Clinical and laboratory diagnosis of acute pancreatitis. Annali italiani di chirurgia. 1995, vol. 66, no. 2, s. 165-170, ISSN 0003-469X.
- ↑ RACEK, Jaroslav, et al. Klinická biochemie. 2. vydání. Praha : Galén, 2006. 329 s. s. 87. ISBN 80-7262-324-9.
- ↑ BERMEJO, JF, J CARBONE a JJ RODRIGUEZ, et al. Macroamylasaemia, IgA hypergammaglobulinaemia and autoimmunity in a patient with Down syndrome and coeliac disease. Scandinavian journal of gastroenterology. 2003, vol. 38, no. 4, s. 445-447, ISSN 0036-5521.
- ↑ LAWSON, GJ. Prevalence of macroamylasaemia using polyethylene glycol precipitation as a screening method. Annals of clinical biochemistry. 2001, vol. 38(Pt 1), s. 37-45, ISSN 0004-5632.
- ↑ VENTRUCCI, M, A CIPOLLA a M MIDDONNO, et al. Macroamylase detection in serum using selective precipitation: a rapid and reliable assay. Italian journal of gastroenterology and hepatology. 1999, vol. 31, no. 9, s. 846-849, ISSN 1125-8055.
- ↑ CUTOLO, M, A SULLI a A BARONE, et al. Macroamylasemia: a possible cause of unexplained hyperamylasemia in rheumatoid arthritis. British journal of rheumatology. 1995, vol. 34, no. 3, s. 290-292, ISSN 0263-7103.
- ↑ HORTIN, GL, AL SUMMERFIELD a TR WILHITE, et al. Detection of autoantibodies to amylase by ELISA: comparison of detection of macroamylase and free autoantibody. Clinical chemistry[online]. 1994, vol. 40, no. 12, s. 2254-2259, dostupné také z <http://clinchem.aaccjnls.org/content/clinchem/40/12/2254.full.pdf,>. ISSN 0009-9147.
- ↑ MORIYOSHI, Yuriko, Tadashi TAKEUCHI a Keiko SHIRATORI. Fecal isoamylase activity in patients with pancreatic diseases. Pancreas. 1991, vol. 6, no. 1, s. 70-76, ISSN 0885-3177.
- ↑ GROMASHEVSKAIA, LL, GF STREMETSKII a TV BOGATYR'. Opredelenie izofermentov amilazy v kale. Laboratornoe delo. 1981, roč. 1981, no. 10, s. 609-612, ISSN 0023-6748.
- ↑ MÜLLER, G. Fakale Enzymdiagnostik. Deutsche Zeitschrift für Verdauungs- und Stoffwechselkrankheiten. 1979, vol. 39, no. 6, s. 257-265, ISSN 0012-1053.
- ↑ HINTNER, I, G MÜLLER a A THEUNE. Bestimmungen von Isoenzymen der alkalischen Phosphatase und alpha-Amylase im Stuhl. Zeitschrift für die gesamte innere Medizin und ihre Grenzgebiete. 1977, roč. 15, vol. 32, no. 12, s. 292-295, ISSN 0044-2542.
Links
Source
- with the permission of the author taken from KOCNA, Petr. GastroLab : MiniEncyklopedie laboratorních metod v gastroenterologii [online]. ©2002. The last revision 2011-01-08, [cit. 2011-03-04]. <http://www1.lf1.cuni.cz/~kocna/glab/glency1.htm>.