Gel permeation chromatography
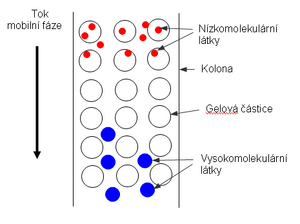
It is based on the different permeability holes and hollow niches on the particles of the stationary phase for different-sized particles of the partitioned mixture.
There are many varying-sized tunnels and caves in the stationary phase.
It is possible, however, for some components of the divided mixture to have such large particles that they won't fit, allowing them to flow only around the balls of the separating material. Thus, it is entrained the fastest since it has the smallest available space during the entrainment of the mobile phase.
Another component may have particles of such a size that they already fit into at least some of the openings in the beads of the separation material. Thus, during the flow, these particles have available not only the space around the balls but also the space of depressions of sufficient size, where they can diffuse freely. As a result, their speed (with a constant mobile phase flow rate, of course) is slightly slower than that of the large particles of the first considered component.
available for their movement space of all the openings in the balls of the separating materials component will therefore move the slowest.
In the final effect, theoretically, the first component with the largest particles and the last component with the smallest particles will flow out of the chromatographic column. Therefore, gel permeation chromatography is used to separate mixtures of high-molecular substances (mainly proteins) according to their molecular weights.
But a necessary condition is that the material of the separation medium to all the separated components is inert and does not specifically retain any of them.
If we want to separate a mixture as well as possible, we must choose a stationary phase with the most appropriate range of particle sizes. Such selection, therefore, depends on the expected size range (molecular weight) of the components of the split mixture. Fortunately, we have an extensive range of commercial materials at our disposal, and we can normally separate mixtures of relatively low-molecular substances with a molecular weight of the order of thousands to tens of thousands of daltons as well as mixtures of very high-molecular substances with molecular weight of hundreds of thousands to a million.
According to the polarity of the stationary and mobile phases, we distinguish between hydrophilic and hydrophobic. In the vast majority, however, we encounter the first ones, i.e. hydrophilic systems, and among them, the system of polysaccharide skeletons as the stationary phase and aqueous solutions as the mobile phase ultimately prevails.
Gel permeation chromatography is sometimes incorrectly called molecular filtration or molecular sieve filtration. As the true nature of gel permeation chromatography implies, these names are misleading because there is no filtration involved.