Prenatal diagnosis
Prenatal diagnosis represents a set of methods and procedures used to diagnose an unborn individual. Prenatal diagnosis requires an interdisciplinary approach, in which clinical genetics, gynecology and obstetrics, clinical biochemistry and imaging methods. As a follow-up to the diagnosis itself, interdisciplinary cooperation is even broader. Prenatal diagnosis can be considered a part of fetal medicine, which includes not only diagnosis but also treatment (prenatal therapy).
Tasks of prenatal diagnosis[edit | edit source]
Prenatal diagnostics enables us to diagnose a number of diseases and pathologies in an unborn human individual. The main task of prenatal diagnosis is the (as early as possible) diagnosis of these pathological conditions. Based on the established diagnosis, we can:
- inform the mother (parents) about the diagnosis of the fetus, its prognosis (both during pregnancy and in the postnatal period) and the next possible procedure,
- take specific measures for the further course of the pregnancy, for the management of the birth (birth by caesarean section) or for follow-up care (birth in a special center with a follow-up to acute surgical or other treatment),
- start prenatal therapy of the fetus (rarely - for example, solving obstructive defects of the urinary system using shunts, intraumbilical transfusion in anemia of the fetus),
- in the case of an unfavorable diagnosis, it is possible in the Czech Republic in accordance with the current legal norm (Act 66/1986 Coll. – On artificial termination of pregnancy) to artificially terminate a pregnancy for genetic reasons, up to the 24th week of pregnancy . In the case of an extremely unfavorable diagnosis (disease incompatible with life - e.g. anencephaly), the same law allows artificial termination of pregnancy at any time (even after the 24th week of pregnancy).
The availability of high-quality screening for all pregnant women and the possibility of targeted prenatal diagnosis in high-risk pregnancies makes it possible to increase prenatal detection of selected types of chromosomal syndromes and birth defects in the population.
Indications for genetic consultation[edit | edit source]
As part of comprehensive care for the pregnant woman and the fetus, a genetic consultation is also necessary in certain cases. The attending physician (gynecologist-obstetrician) sends a pregnant woman to the clinical genetics workplace especially in the following cases:
- pregnant with the occurrence of congenital developmental defects or hereditary diseases in family, respectively in personal anamnesis (of course also applies in case of occurrence of such diagnoses in the father of the child or in his family),
- pregnant with a positive result of prenatal screening examination (combined screening of the first trimester, biochemical screening of the second trimester, or integrated screening),
- pregnant with an abnormal finding on an ultrasound examination of the fetus (direct signs of VVV or other fetal abnormalities, abnormalities in the amount of amniotic fluid, etc.),
- pregnant from 35 years of age and older (so-called age indication); the risk of chromosomal aberrations increases continuously with increasing age of the mother (the limit of 35 years was set artificially).
During the genetic consultation, the pregnant woman may be offered a more detailed prenatal diagnosis (invasive methods, detailed ultrasound examination, fetal echocardiography, fetal MRI). In the case of a confirmed diagnosis, the pregnant woman returns to the clinical geneticist to consider the next procedure.
Non-invasive prenatal diagnosis[edit | edit source]
Non-invasive methods of prenatal diagnosis are well suited for screening and are used to examine all pregnant women. Regular prenatal care includes regular ultrasound examinations (I. up to the 14th week, II. in the 20th - 22nd week, III. in the 30th - 32nd week). For the screening of the most common morphological and chromosomal congenital defects of the fetus, a combined (biochemical and ultrasound) screening in the first trimester of pregnancy is preferred, which, however, is not yet covered by public health insurance funds.[1] [2] Recently, the possibilities of prenatal screening have been expanded to include non-invasive prenatal testing (NIPT) from the venous blood of the pregnant woman. This is an examination of the free DNA of the fetus that circulates in the mother's blood. This examination is not covered by the insurance company
Combined First Trimester Screening[edit | edit source]
![]() Some information may be out of date, see: Congenital Developmental Defects Screening for a new procedure.
Some information may be out of date, see: Congenital Developmental Defects Screening for a new procedure.
Combination:
- biochemical blood screening - examination of beta free HCG subunit and PAPP-A (pregnancy-associated plasma protein) in the 11th week and
- first-trimester ultrasound screening - examination of the thickness of the nuchal translucency (NT) and the presence of the nasal bone (NB) in the 13th week; the risk of chromosomal aberrations (Down syndrome, Edwards syndrome and Patau syndrome) increases sharply with NT > 2.3 mm.[3]
In case of a positive result, a genetic consultation is recommended with the possibility of further examinations (cfDNA, CVS, AMC).
Examination of biochemical markers (triple test)[edit | edit source]
The mother's blood is taken for the screening of biochemical markers after the 16th completed gestational week. It is a so-called triple test - levels are examined in the maternal serum
- alpha-fetoproteinu (AFP),
- chorionic gonadotropin (hCG)
- and unconjugated estriol (uE).
Elevated AFP values are a marker of congenital defects of the fetus without skin covering - eg spinal cleft, decreased AFP values and elevated hCG values are characteristic of fetuses with Down syndrome. uE reflects the overall risk of pregnancy. For a more accurate assessment, level deviations are expressed in multiples of medians (MoM), and for their evaluation, computer programs were developed that evaluate not only the length of pregnancy, but also the age of the pregnant woman, her weight, and are based on the medians of the respective laboratory.
Invasive examination is recommended if the screening is positive, i.e. at risks higher than 1:350. A negative screening result means that the risk of the defects being sought is lower than the risk that is the reason for an invasive examination.
Quadruple test: AFP + hCG + uE3 + inhibin A (inhA) at 15-18 weeks.[4]
Integrated test[edit | edit source]
Combination of combined first trimester screening and triple test.
Ultrasound examinations[edit | edit source]
Ultrasound examinations at the 6th, 13th, 20th and 32nd weeks of pregnancy are part of standard care for pregnant women. If abnormalities are detected, the pregnant woman should be sent for a consular ultrasound examination and possibly also for a genetic consultation.
Indirect signs of fetal impairment include retardation of development, too little or too much amniotic fluid, disproportion of the development of individual parts of the fetus and are indications of chromosomal examination.
Targeted ultrasound examination is still the most effective method of prenatal diagnosis. It makes it possible to diagnose in the fetus, for example, head development disorders (anencephaly), spinal clefts, heart defects, kidney defects ([[renal agenesis|agenesis] ], polycystosis), urinary tract atresia, Turner's syndrome, with less certainty can be detected, for example, cleft lip, GIT obstruction, minor reduction deformities of the limbs.
In 13.-14. week of pregnancy, an ultrasound measurement of the nuchal fold - nuchal translucency (NT) is performed. Using US, the thickness of the anechoic zone in the nuchal area of the fetus between the skin and the connective tissue that covers the cervical spine is evaluated. With thickening > 3 mm, there is an increased risk of chromosomal aberration, so prenatal diagnosis of the fetal karyotype (amniocentesis) is indicated.
In the 18.-20. week, measurement of fetal size (biparietal diameter, head circumference, femur length), nuchal translucency and detection of congenital somatic defects are carried out, so that an abortion can be performed by the 24th week (culture for amniocentesis takes 3 weeks).
At the 30th week the size and position of the fetus is measured, placenta praevia is ruled out.
Non-invasive prenatal testing (NIPT, NIPS, cfDNA)[edit | edit source]
Examination of free fetal DNA from maternal venous blood. The highly accurate screening method, which is used to detect aneuploidies (trisomy 21, 18, 13 and aneuploidy of sex chromosomes), also makes it possible to determine gender. The spectrum of genetic diseases that can be examined by NIPT is still expanding (microdeletions/microduplications, monogenic diseases). It can be performed from the 10th week of pregnancy, it is not covered by the insurance company.[3]
Currently, there are a number of tests on the market from different manufacturers: Clarigo (manufacturer: Multiplicom), Panorama (Natera), Prenascan (BGI Health Europe), Harmony (Ariosa Diagnostic), cffDNA Gennet, etc.
Invasive prenatal diagnosis[edit | edit source]
The main purpose of invasive examinations is to obtain a tissue sample of the fetus for examination of the karyotype or for molecular genetic examination with the aim of ruling out [[chromosomal aberrations] in the fetus ]] or genetically conditioned diseases. Due to the certain risk associated with their implementation (and also due to the price of these methods), these methods are offered only on the basis of a special indication (see e.g. indication of chromosomal examination).
Amniocentesis[edit | edit source]
Amniocentesis (AMC) involves taking a sample of amniotic fluid with a needle through the abdominal wall under ultrasound control. It is usually performed between the 16th and 18th week of pregnancy. It enables examination of cultured cells and uncultured cells of amniotic fluid and its biochemical examination. The karyotype of the fetus can be examined from the cells of the amniotic fluid after 10-20 days of cultivation, the biochemical examination of the amniotic fluid is mainly aimed at evaluating the level of AFP. The risk of miscarriage after the procedure is less than 1%.
Collection of chorionic villi[edit | edit source]
Chorionic villus sampling (CVS) is performed earlier than amniocentesis, roughly between the 10th and 13th gestational weeks. The collection of chorionic tissue is performed with a special needle under ultrasound control, most often transabdominally, less often transcervically (not in the Czech Republic). The advantage of chorionic villus sampling (CVS) compared to amniocentesis is the possibility of earlier diagnosis (for example, following the first-trimester screening for developmental defects). The culture of chorionic cells (for cytogenetic examination) is also faster (trophoblast cells have high mitotic activity and the karyotype can be examined after the addition of colcemid after 1-2 hours) than the culture of amniocytes obtained by amniocentesis. The risk of the procedure is the same as in the case of amniocentesis (risk of pregnancy loss 0.5-1%). A certain disadvantage of CVS is the risk of placental mosaicism, which can be a source of diagnostic uncertainty. The detected chromosomal abnormalities must therefore still be confirmed by examination of the amniotic fluid, as the finding in the trophoblast cells does not necessarily mean the same involvement of the fetal tissues.
Genetic testing[edit | edit source]
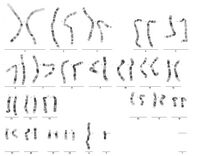
Molecular genetic examinations after AMC or CVS:
- karyotype (classical cytogenetics): cultivation in nutrient medium - proliferation of cells, addition of colchicine - arrest of cell division in metaphase stage, staining with Giemsa dye, microscopic examination, compilation of karyogram using a computer; lasts 10 - 14 days;[5]
- QF-PCR (quantitative fluorescence PCR) enables rapid prenatal detection of the most common aneuploidies in chromosomes 13, 18, 21, X and Y; polymerase reaction with fluorescently labeled primers allows specific markers to be separated and quantified on an automated genetic analyzer; the mother's DNA is also analyzed in this way for comparison; the advantage is accuracy and speed - results within the 2nd day.
- array CGH (microarray-based comparative genomic hybridization) principle is the comparison of control DNA from a healthy person with DNA tested; DNA is labeled with fluorescent dyes, followed by image analysis by a computer and interpretation of the result by a laboratory worker; the goal is the detection of small unbalanced rearrangements on chromosomes that are not captured by conventional cytogenetics by G-banding; does not capture chromosomal aberrations without material gains and losses; is very sensitive to the quality of the input material.[6]
- SNP array (single nucleotide polymorphism): whole-genome screening with 1000 times more discriminating power than a classic karyotype; detects submicroscopic changes at the 10 kb resolution level using the presence of single nucleotide polymorphisms in the patient's genome; uses statistically selected single nucleotide polymorphism (SNP) sites to diagnose changes in the genome; there is no need for cultivation, the examination follows the direct isolation of DNA from the native tissue (amplification, fragmentation, precipitation and resuspension of DNA, application to the examination chip for hybridization with the present examination oligonucleotides, hybridization, and indication of the hybridized fragments, computer evaluation - comparison of the patient's data with a defined group data obtained from a comparison group of 200 individuals); cannot detect balanced reconstructions and mosaics < 10%.
Cordocentesis[edit | edit source]
Cordocentesis (CC) or umbilical cord puncture is another invasive method. The examination can only be carried out relatively later - generally from the 18th gestational week [7] Puncture of the umbilical cord and collection of fetal blood from the umbilical vein is performed with a special needle under ultrasound control. The obtained blood elements (lymphocytes of the fetus) can be used again for examination of the karyotype of the fetus or for molecular genetic examination. Fetal lymphocyte karyotyping is very fast, results are available within 48-72 hours[7]. Cordocentesis can thus be successfully used for repeated - rapid examination of the karyotype, if the previous sampling with culture (amniocentesis, CVS) failed or yielded ambiguous results. Fetal blood is a valuable diagnostic material that can be used, among other things, to determine the blood group of the fetus, diagnose fetal alloimmunization, fetal infection or to diagnose certain hereditary diseases and disorders (e.g. hemoglobinopathy) [7]. Of course, it also enables immunological (antibodies) and biochemical (ions) tests.
The risk of the procedure in experienced hands is comparable to that of amniocentesis (under 1% fetal loss)[7]. The puncture of the umbilical cord is used as an approach within the framework of intraumbilical transfusion, which is a therapeutic procedure (prenatal therapy) carried out for example in case of alloimmunization of the fetus (often incompatibility in the Rh system).
Fetoscopy[edit | edit source]
The examination consists of introducing an optical system (fetoscope) into the amniotic cavity through a small incision in the abdominal wall. It enables the visualization of the fetus and the collection of a tissue sample (skin, muscles, liver) for further examination (suspected hereditary diseases or defects, if DNA diagnosis is not possible). It is most often performed in the 18.-20. week, performance risk 3-10%. Precisely because of the high risk, it is practically not used today as a purely diagnostic method.
Links[edit | edit source]
Related Articles[edit | edit source]
- Screening for congenital developmental defects ▪ Congenital developmental defects ▪ At-risk pregnancy and newborn
- Clinical genetics ▪ Indications for chromosomal examination ▪ Preimplantation genetic diagnosis
- Monogenically inherited diseases ▪ Chromosomal aberrations
References[edit | edit source]
- ↑ ČGPS ČLS JEP,. Regular ultrasound examinations during prenatal care. Collection of recommended practices No. 3/2019 [online]. 2019, y. -, vol. -, p. -, Available from <https://www.gynultrazvuk.cz/data/clanky/6/dokumenty/2019-03-pravidelna-uz-vysetreni-v-prubehu-prenatalni-pece-dp-cgps-cls-jep-revize.pdf>.
- ↑ ČGPS ČLS JEP,. Principles of dispensary care during pregnancy. Collection of recommended practices No. 1/2019 [online]. 2019, y. -, vol. -, p. -, Available from <https://www.gynultrazvuk.cz/data/clanky/6/dokumenty/2019-01-zasady-dispenzarni-pece-v-tehotenstvi-dp-cgps-cls-jep-revize.pdf>.
- ↑ a b https://www.sanus.cz/dalsi-obory/prvotrimestralni-screening-kombinovany-test
- ↑ MESSERLIAN, G M. Laboratory issues related to maternal serum screening for Down syndrome [online]. UpToDate, ©2019. The last revision 2020-04, [cit. 2020-09-28]. <www.uptodate.com>.
- ↑ http://video.muni.cz/public/IBA/portal/karyotyp_w.mp4
- ↑ https://www.clg.cz/vysetreni-plodove-vody/
- ↑ a b c d CALDA, Pavel, et al. Ultrazvuková diagnostika v těhotenství: pro praxi. 1. edition. Praha : Aprofema, 2007. 268 pp. ISBN 978-80-903706-1-6.
References[edit | edit source]
- CALDA, Pavel, et al. Prenatal diagnosis and treatment of the fetus. 1. edition. Prague : Levret, 1998. 129 pp.
- CALDA, Pavel, et al. Ultrasound diagnostics in pregnancy: for practice. 1. edition. Prague : Aprofema, 2007. 268 pp. ISBN 978-80-903706-1-6.
- KOČÁREK, Eduard – PÁNEK, Martin – NOVOTNÁ, Drahuše. Clinical cytogenetics I.: introduction to clinical cytogenetics , investigative methods in clinical cytogenetics. 1.. edition. Prague : Karolinum, 2006. 120 pp. ISBN 80-246-1069-8.
External links[edit | edit source]
- Actual Gynecology and Obstetrics, online open access journal
- Gynstart - information portal for gynecologists
- International Society for Prenatal Diagnosis
- [Mefanet http://mefanet.lfp.cuni.cz/clanky.php?aid=218%7CImportance of ultrasonography in pregnancy – MEFANET]
- Examination of the karyotype of the fetus from amniotic fluid cells (video)

