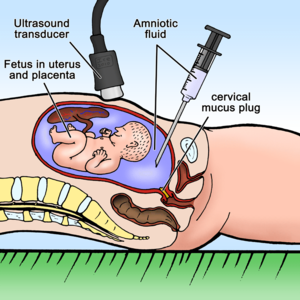
Amniocentesis
Amniocentesis (AMC, collection of amniotic fluid) is the most common invasive method used in prenatal diagnosis, in which a small amount of amniotic fluid is taken transabdominally with cells. The sample will further be used in the examination of suspected congenital developmental defects or diseases of the foetus.
Indication[edit | edit source]
In indicated cases, the collection is proposed by a doctor in a genetic outpatient clinic and the patient has the right to decide whether to undergo it. Amniocentesis is performed in specialized outpatient clinics of gynecological departments. There are a number of indications for performing amniocentesis:
- Abnormal finding during another type of examination during pregnancy is also a very common indication (e.g. abnormal biochemical screening, abnormal ultrasound finding, high value nuchal translucency during examination in the 1st trimester of pregnancy, etc.).
- Another of the more common indications is the so-called age indication. Genetic consultation is automatically recommended for pregnant women over 35 years of age (because of the higher probability of the occurrence of Numerical chromosomal abnormalities in the fetus, especially Down syndrome)[1]. Women in this age have the right to undergo invasive examinations (including amniocentesis) only from this age indication, regardless of the results of other examinations.
- Furthermore, due to the occurrence of hereditary diseases in the family (e.g. cystic fibrosis, hemophilia, etc.). Amniocentesis (and other invasive methods) can be used to obtain a fetal sample for molecular genetic testing (see below).
- Amniocentesis is important in a number of other cases: for example, due to repeated miscarriages in previous pregnancies, in the event of balanced chromosomal aberrations in one of the parents, in the event of structural developmental defects in the fetus and in other indicated cases.
- From a historical point of view, amniocentesis was also performed to determine the gender of the fetus in case of X-linked hereditary diseases in the family (hemophilia, muscular dystrophy). However, this indication of amniocentesis and selective sex reduction in X-linked diseases has now been completely replaced by molecular genetic diagnostics.
In the Czech Republic, amniocentesis is still sovereignly the most frequently performed method of invasive prenatal diagnosis, with a significant lead over CVS (2nd place) and cordocentesis[2].
Examination[edit | edit source]
Amniotic fluid is collected from 15-16. gestational week of pregnancy. At this time, there is a physiologically greater amount of amniotic fluid in the amniotic sac (207 ± 92 ml[3]), therefore, the procedure can be performed well and the risk of fetal injury is minimized. Theoretically, it is possible to perform the procedure earlier (the so-called early amniocentesis), but a smaller amount of fluid can be obtained (from the 11th to the 14th week of pregnancy, it is possible to take only as many milliliters of amniotic fluid as the gestational age of the fetus in weeks[4]).
The sampling itself is performed on an outpatient basis. The woman lies on her back, the doctor first ultrasound examination determines the position of the fetus and placenta. Next, it disinfects the selected place on the skin. Under constant ultrasound control (so as not to injure the fetus), he performs an injection with a thin needle, which is guided transabdominally through the wall of the uterus and through the amniotic sac. About 20 ml of amniotic fluid' is drawn into the syringe from the amniotic cavity. The procedure is subjectively described as unpleasant, but painless (comparable to blood sampling). Sometimes a „dull pain“ is felt.
Processing and results[edit | edit source]
After the amniotic fluid is aspirated, the cells are separated from it by centrifugation. The investigation is carried out in several ways:
Cytogenetic examination[edit | edit source]
After separation, the amniotic fluid cells are further cultured in a culture medium where they multiply, then they are fixed and stained. Cell culture takes approximately two weeks. This is followed by an examination of the karyotype under an optical microscope, which is primarily intended to rule out the most common numerical chromosome aberrations - such as Down syndrome, Patau syndrome, Edwards syndrome Turner syndrome, Klinefelter syndrome . Examination under an optical microscope can also draw attention to large chromosomal rearrangements (translocations, inversions) or large deletions and duplications. However, it is not suitable for identifying small rearrangements, for example microdeletion syndromes. If amniocentesis was performed for suspected microdeletion syndrome, it is advisable to supplement (or even replace) karyotyping with a microarray type examination (see below).
Molecular genetic examination[edit | edit source]
It is also possible to isolate DNA from fetal cells, which can then be targeted for the presence of various mutations. Such an examination is carried out targeted, based on the identification of a specific mutation in the parents (e.g. prenatal diagnosis cystic fibrosis of the fetus in a couple where both father and mother are heterozygous carriers of the mutation).
Today, the so-called QFPCR (also known as amnioPCR in connection with amniocentesis) is already a routine method. This PCR modification is used as a so-called "rapid diagnosis" in the verification of suspected chromosomal aneuploidy in the fetus. The examination is based on the quantitative evaluation of the amplification of certain markers on selected chromosomes (typically on chromosomes 13, 18, 21, X and Y, which are the chromosomes associated with the most significant aneuploidies)[5]. The advantage over the classic karyotype examination is the speed of this method (results available within a few days), however, the method is relatively narrowly focused on the above-mentioned aneuploidy and thus cannot replace the classic cytogenetic examination in all directions.
Molecular cytogenetic examination[edit | edit source]
Currently, it is common to examine, in indicated cases, samples obtained by amniocentesis and molecularly cytogenetically, most often using the microarray method (e.g. Array CGH)[6]. Indications for the use of this method vary, some workplaces supplement simple karyotyping with the microarray method when an ultrasound finds a developmental defect in the fetus, in some workplaces the microarray method replaces karyotyping completely.
The FISH method is now used rather rarely in prenatal diagnostics.
Biochemical analysis[edit | edit source]
Currently, biochemical analysis of amniotic fluid is no longer used in practice, as a detailed ultrasound examination is fully sufficient for the diagnosis of structural developmental defects, and for the diagnosis of monogenic diseases it is more advantageous to proceed directly to molecular-genetic diagnosis.
From a historical perspective, it is possible to mention the examination of various biochemical analytes (markers), such as α-fetoprotein, acetylcholinesterase and γ-glutamyltransferase. By determining the level of alpha-fetoprotein (AFP) and acetylcholinesterase (AChE), it is possible to reveal disorders of the integrity of the fetal body - open defects of the neural tube, disorders of the integrity of the abdominal wall. Decreased levels of γ-glutamyltransferase (GGT) are associated with cystic fibrosis[7].
Delivery of results[edit | edit source]
The results of the examinations performed (most often the result of the karyotype of the fetus) are communicated by the clinical geneticist. It is completely inappropriate to communicate the results without a follow-up to appropriate genetic counseling (there is a risk of misunderstanding or even misinterpretation of the results). The geneticist should explain in detail not only the specific findings, but also their meaning. Even a normal karyotype does not mean 100% certainty that the child will be born completely healthy[8]. If the results of the amniocentesis show a certain pathology of the fetus, it is necessary to carefully explain the nature and prognosis of such a condition to the pregnant woman (parents) and offer other possible examinations or measures. In the case of confirmation of a serious (and incompatible with normal postnatal development) diagnosis of the fetus, it is currently possible in the Czech Republic (in accordance with current legislation) to terminate the pregnancy prematurely up to the completed 24. week of pregnancy. All decisions about further examinations, procedures and eventual termination of pregnancy are fully within the competence of the pregnant woman. A clinical geneticist can - in accordance with the non-directive philosophy of this field - only inform the pregnant woman about her options and all alternatives, but his role is not (and must not be) to recommend, advise or even force her to do something.
Risks[edit | edit source]
The risk during collection is low and consists in the possibility of short-term outflow of amniotic fluid, bleeding, introduction of infection, and in extreme cases even spontaneous abortion (it is given in the range of 1:200 to 1:100). In general (in every pregnancy even without amniocentesis), the risk of spontaneous abortion after 16 weeks of pregnancy is approximately 3%. There is no evidence that amniocentesis is harmful to the further development of the fetus[9].
Links[edit | edit source]
Related articles[edit | edit source]
- Clinical Genetics
- Prenatal diagnosis
- Chorio villus sampling
- Cordocentesis
- Indication of chromosomal examination
- Amniotic fluid
External links[edit | edit source]
- Amniodex interactive website for women deciding whether or not to undergo amniocentesis
References[edit | edit source]
- ↑ GARDNER, R. J. – SUTHERLAND, G. R. Chromosome abnormalities and genetic counseling. 3. edition. Oxford University Press, 2004. 577 pp. ISBN 0195149602.
- ↑ GREGOR, Vladimír – ŠÍPEK, Antonín. Efektivita prenatální diagnostiky v České republice v období 1994–2008. Aktuální gynekologie a porodnictví [online]. 2009, vol. 1, p. 25-29, Available from <https://www.actualgyn.com/pdf/2009_8.pdf>. ISSN 1803-9588.
- ↑ ŘIČÁNEK, Jan. Amniocentéza [online]. [cit. 28.10.2010]. <http://www.igyn.cz/amniocenteza.html>.
- ↑ KOČÁREK, Eduard – PÁNEK, Martin – NOVOTNÁ, Drahuše. Klinická cytogenetika I.: úvod do klinické cytogenetiky, vyšetřovací metody v klinické cytogenetice. 1.. edition. Karolinum, 2006. 120 pp. ISBN 80-246-1069-8.
- ↑ CALDA, Pavel. Ultrazvuková diagnostika v těhotenství: pro praxi. 1. edition. Aprofema, 2007. 268 pp. ISBN 978-80-903706-1-6.
- ↑ RICKMAN, L – FIEGLAR, H – SHAW-SMITH, C. Prenatal detection of unbalanced chromosomal rearrangements by array CGH. J Med Genet [online]. 2006, vol. 4, p. 353–336, Available from <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2563226/pdf/353.pdf>. ISSN 1468-6244. PMID: 16199537.
- ↑ MACEK, M – MACEK, M – STUHRMANN, M. The direct early diagnosis of cystic fibrosis by the detection of the delta F508 CFTR gene mutation in a prematurely delivered boy. Clin Genet [online]. 1991, vol. 39, p. 219-22, Available from <https://www.ncbi.nlm.nih.gov/pubmed/1709842>. ISSN 0009-9163.
- ↑ HURST, J. A. – HELEN, V. F. – HALL, J. G.. Oxford Desk Reference: Clinical Genetics. 1. edition. Oxford University Press, 2005. 752 pp. ISBN 0192628968.
- ↑ KOLEKTIV ÚSTAVU BIOLOGIE A LÉKAŘSKÉ GENETIKY UK 2. LF A FN V MOTOLE,. Amniocentéza [online]. [cit. 28.10.2010]. <http://www.eurogentest.org/index.php?id=160>.
Used literature[edit | edit source]
- KOČÁREK, Eduard – PÁNEK, Martin – NOVOTNÁ, Drahuše. Klinická cytogenetika I.: úvod do klinické cytogenetiky, vyšetřovací metody v klinické cytogenetice. 1.. edition. Karolinum, 2006. 120 pp. ISBN 80-246-1069-8.
- CALDA, Pavel. Ultrazvuková diagnostika v těhotenství: pro praxi. 1. edition. Aprofema, 2007. 268 pp. ISBN 978-80-903706-1-6.
- HURST, J. A. – HELEN, V. F. – HALL, J. G.. Oxford Desk Reference: Clinical Genetics. 1. edition. Oxford University Press, 2005. 752 pp. ISBN 0192628968.