Identification of chromosomes
Chromosome staining[edit | edit source]
Classic coloring[edit | edit source]
Chromosomes stained with Giemsa-Romanowski solution are homogeneously dark. This type of staining makes it possible to evaluate the number and gross structure of chromosomes, breaks and unstable rearrangements (in individuals exposed to clastogenic agents). Classical staining is also used in patients with disorders of repair of damaged DNA (Fanconi anemia, ataxia-telangiectasia, Nijmegen-breakage syndrome).
G bars[edit | edit source]
G banding is the most routinely used stain in cytogenetic laboratories. A cross-striped pattern appears on trypsin-incubated chromosomes after staining with Giemsa-Romanovsky solution. The number of bands depends on the degree of chromosome condensation. In metaphase, there are 400-450 bands on the chromosomes. In prometaphase, around 850 bands are identifiable in the karyotype. heterochromatin regions formed by DNA rich in adenine and thymine stain darkly. Light bars correspond to euchromatinrich in guanine and cytosine.
R bars[edit | edit source]
The R striping method is based on high temperature (87 °C) and subsequent staining with Giemsa-Romanovsky solution. The resulting banding pattern is the reverse of G banding (G negative).
AgNOR staining[edit | edit source]
This staining is used to monitor secondary constriction variants acrocentric chromosomes. Silver nitrate selectively precipitates in the nucleolar organizer region.
C staining[edit | edit source]
The method serves to display constitutive heterochromatin – satellite DNA. In humans, there is variability in the size of constitutive heterochromatin on the long arms of chromosomes 1, 9, 16 and Y.
Special Methods[edit | edit source]
SCE (Sister Chromatide Exchange)[edit | edit source]
Symmetric exchange of DNA segments between sister chromatids observed on metaphase chromosomes. Under physiological conditions, 4–6 SCEs occur with each replication. UV radiation and the action of genotoxic substances induce an increase in the number of SCEs in cells.
The "harlequin" technique, based on the different staining of sister chromatids, is used to visualize SCE. Peripheral blood lymphocytes are cultured in medium with BrdU (5-bromo 2-deoxyuridine – thymine analog). During replication, BrdU is incorporated into nascent DNA strands instead of thymine. In the metaphase of the following cell cycle, the chromosomes in one sister chromatid have BrdU-substituted DNA in both strands, and in the other chromatid one strand is original and the other with BrdU. The presence of BrdU in DNA reduces its ability to bind certain dyes, and after staining with e.g. Giemsa dye, chromatids with BrdU are lighter.
According to the color of the chromatids, it is possible to distinguish the metaphases of the 1st, 2nd and 3rd division in the culture.
The SCE test in vitro on human lymphocytes is used to test the clastogenic effects of substances. This test is more sensitive than ZCHA. In vivo causes an increase in the number of SCEs, e.g. smoking, and some cytostatics (based on alkylating substances). In individuals with Bloom's syndrome, SCE examination is a routinely used investigative method (number of exchanges and more than 100 per mitosis).
Fragile-X[edit | edit source]
A number of fragile places are known in the human karyotype - areas of frequent occurrence of breaks. They appear on metaphase chromosomes after exposure to certain substances or when growing in deficient media. In most cases, their presence is not related to a pathological phenotype. A certain exception is the fragile site Xq27.3 (FRAXA) in patients with fragile X.
Xq27.3 is a folate-sensitive site, crere can be visualized after cultivation in a medium with a low content of folic acid, or in the presence of acid leaf FudR antagonists (5'-fluoro 2'-deoxyuridine) or methotrexate.
Cytogenetically, under optimal laboratory conditions (and with a bit of luck), the presence of fra Xq27.3 can be diagnosed in both affected men and carrier women.
Fragile X syndrome is caused by the expansion of a CGG triplet repeat in the first exon of the FMR1 (Fragile X Mental Retardation 1) gene. Currently, cytogenetic diagnostics are no longer used for the diagnosis of FRAXA and preference is given to direct DNA diagnostics focused on the number of repetitions in a given gene.
Another folate-sensitive fragile site lies in the Xq28 region; FRAXE - associated with milder mental retardation and possibly benign FRAXF.
HRT (High Resolution Technique)[edit | edit source]
The HRT method makes it possible to analyze very long, only partially spiraled chromosomes. A special culture technique involves synchronizing cell culture division with methotrexate and then arresting division in prometaphase. Up to 850 G-bands can be identified on chromosomes. This method is suitable for the analysis of small-scale structural rearrangements (e.g. microdeletions), but it is no longer used in routine diagnostics, as it has been completely replaced by the (even more accurate) microarray method.
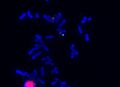
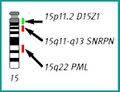
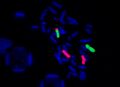
FISH (Fluorescent in situ hybridization)[edit | edit source]
The FISH method uses DNA probes labeled with a fluorescent dye to detect specific stretches of DNA. FISH can be used as a complementary method in cytogenetic examination. Probes are applied directly to cellular material (chromosomes in metaphase and prometaphase or interphase nuclei), DNA isolation is not necessary. The preparations are evaluated under a fluorescence microscope.
FISH - locus specific probe, Prader-Willi
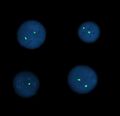
Interphase FISH - α-satellite probe, mosaic 46XX/45X
Probe Types[edit | edit source]
- Locus-specific probes can be used for nuclei in interphase as well as for metaphase chromosomes. It is used to investigate microdeletions, subtelometric rearrangements and amplifications.
- Satellite (centromeric, telometric) probes can be used for nuclei in interphase as well as for metaphase chromosomes. They are used to determine the number of individual chromosomes and to investigate mosaicism.
- Whole chromosome (painting) probes are only suitable for metaphase chromosomes. Used to evaluate chromosome rearrangements.
Most commonly used fluorescent dyes[edit | edit source]
- DAPI (4',6-diamidino-2-phenylindole) – for staining preparations (counterstaining).
- FITC (fluorescein-5-isothiocyanate) – green signal.
- TRITC (tetramethylrhodamine-5-isothiocyanate) – red signal.