Metabolic bone disease of immaturity
Metabolic bone disease of immaturity (MBD) or osteopenia, osteomalacia or rickets in immaturity is a disorder of the bone mineralization process that affects at-risk premature infants. It includes a disorder in the formation of organic bone matrix (or osteoid) as well as a disorder in its own mineralization, i.e. the incorporation of minerals (calcium, phosphorus and others) into the newly synthesized osteoid.
The greatest part of the bone mineralization of the fetal skeleton takes place in the third trimester of pregnancy, with a maximum shortly before birth itself. Therefore, MBD mainly affects newborns born before the 28th week of pregnancy and newborns with other risk factors (repeated infections, severe form of bronchopulmonary dysplasia, significant bowel resection, etc.). Loss of fetal accretion of calcium and phosphorus, earlier onset of bone remodeling, insufficient supply of minerals (osteomalacia/rickets) and impaired bone matrix formation (osteopenia) contribute to the development of metabolic bone disease from immaturity. Due to the absence of widely accepted diagnostic criteria, the exact incidence of MBD is debatable. MBD is accompanied by hypophosphataemia (P < 1.8 mmol) and hyperphosphataemia (ALP > 500 IU/l).[1] Clinical signs begin to appear between 5 and 11 weeks of age and include increased work of breathing secondary to chest instability caused by softening (or fractured) ribs, enlargement of cranial sutures, unusual prominence of the forehead ("frontal bossing"), fractures, and postnatal growth failure. Among the short-term consequences of MBD are bone fractures (ribs, humerus or femur) usually between 3 and 6 months of uncorrected age. Long-term consequences of MBD include a tendency to lower body height and decreased bone density, and may be a risk factor for the development of early osteoporosis. Early identification of at-risk newborns, consistent monitoring of laboratory parameters of bone metabolism and adequate individualized supplementation of individual nutrients are important (breast milk does not sufficiently cover the nutritional needs of premature babies).[2][3]
Osteomalacia is a disorder of the physiological process of mineralization, i.e. the incorporation of minerals into the newly formed osteoid. Unmineralized osteoid causes "softening" of the bone, which may present clinically as dolichocephalic flattening of the skull in the newborn. If the mineralization disorder also affects the growth cartilages, it is rickets. It is characterized by a blurred/irregular border between the growth cartilage and the metaphysis on X-ray of the wrist. The development of osteomalacia/rickets is mainly caused by an insufficient supply of calcium, phosphorus and vitamin D. During the period of open growth cartilages, both conditions (osteomalacia and rickets) occur simultaneously.
Osteopenia is a reduced amount of bone tissue, i.e. reduced thickness of compact bone and reduced thickness or number of cancellous bone beams. Unlike osteomalacia, the remaining osteoid is mineralized normally. In premature newborns, osteopenia is caused by insufficient formation or increased resorption of osteoid. Osteopenia occurs as a result of some systemic diseases, administration of some drugs (corticosteroids, furosemide) or as a result of insufficient mechanical stimulation.[2]
Pathophysiology[edit | edit source]
Bone formation: chondrogenic - from cartilage; desmogenous - from ligaments (skull, maxilla, mandible). It includes the formation of an organic bone matrix (or osteoid) and its mineralization, i.e. the incorporation of minerals (calcium, phosphorus and others).
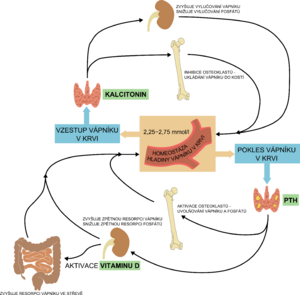
Vitamins (A, C, D), cytokines, minerals and hormones (thyroid hormones, estrogen, calcitonin, growth hormone, parathormone-like peptide - PTHrP) are used in bone formation. Prenatally, active transport of Ca, P and Mg occurs through the placenta. About 99% of the body's calcium and 80% of phosphorus are incorporated into the skeleton at birth at term, this transfer of minerals into the skeleton occurs between 25 weeks of pregnancy and the term of delivery. Postnatally, these minerals are absorbed passively and then actively from the intestine.[1][4]
MBD: biochemical signs of mineral metabolism disturbance → reduced mineralization → abnormal bone remodeling and reduced linear growth rate → osteopenia (↓ matrix) and osteomalacia (↓ minerals) → fractures of ribs and long bones, craniotabes and others.[5]
Biochemical changes in MBD:
- decrease in serum phosphorus level, hypophosphaturia and increased tubular reabsorption of phosphorus;
- phosphorus deficiency → hypercalcemia and hypercalciuria; urinary calcium:phosphorus ratio (mmol/L) > 1.[5]
Risk factors[edit | edit source]
The etiology of metabolic bone disease in premature newborns is multifactorial, the most important factor being insufficient nutrition.
In the last trimester of pregnancy, calcium and phosphorus are deposited in the bones of the fetus, therefore severely premature babies are most at risk of MBD. The placenta plays a key role in this deposition of minerals in the skeleton, therefore, if its function is impaired, there is a risk of developing MBD. Another risk factor is the need for long-term parenteral nutrition, because calcium and phosphorus are poorly soluble in the solutions used, so they cannot be supplied in sufficient quantities. Physical activity plays a major role in bone mineralization, as the development of the skeleton is determined by functional requirements.
Certain medications are involved in the development of MBD when administered long-term. Corticosteroids inhibit osteoblast function, and also reduce intestinal absorption and tubular reabsorption of calcium (this leads to calciuria with an increased risk of nephrocalcinosis). Furosemide and other loop diuretics increase renal Ca losses and thus lead to a negative balance (thiazide diuretics have the opposite effect). Aminophyllines (caffeine, syntophylline) also lead to increased urinary Ca loss, however, the significance is considerably lower than steroids and furosemide. Long-term administration of heparin is also associated with a decrease in bone density. Heparin reduces osteoblast activity, leads to abnormal vitamin D metabolism and secondary hyperparathyroidism. Long-term use of phenobarbital (and/or phenytoin) accelerates the degradation of vitamin D, which may lead to insufficient absorption of Ca.[2]
Prenatal risk factors
- Preeclampsia
- Chorioamniitis, placental infection
- Intrauterine growth restriction[2]
- chronic administration of magnesium to the mother during pregnancy (may cause early osteopenia).[5]
- Postnatal risk factors
- Low gestational age
- Very and extremely low birth weight
- Nutritional disorders: delayed achievement of full enteral intake, long-term parenteral nutrition, vitamin D deficiency
- Aluminum contaminated parenteral nutrition solutions
- Medications (corticosteroids, furosemide, caffeine, syntophylline, heparin, phenobarbital)
- Neonatal morbidity: bronchopulmonary dysplasia, necrotizing enterocolitis, congenital malformations of bowel leading to resection/enterostomy, cholestasis, recurrent infections
- Lack of mechanical stimulation/immobility: long-term need for sedation, relaxation, brain pathology.[2]
Clinical image[edit | edit source]
MBD usually manifests between 6 and 12 weeks of age and is often asymptomatic. Severe forms of MBD are manifested by:
- failure to thrive and growth failure,
- findings reminiscent of rickets (rickets-like) - growth retardation, prominence of the forehead ("frontal bossing"), craniotabes (softening and thinning of the skull bones occipitally and/or parietal), rachitic rosary (prominence of the costochondral junction), enlargement of the epiphyses;
- fractures,
- breathing difficulties due to increased compliance of the chest wall;
- myopia from immaturity - a consequence of the change in the shape of the skull during osteopenia.[1]
Diagnosis[edit | edit source]
Diagnosis is based on the identification of risk factors, clinical status, dynamics of biochemical markers and imaging examinations.
Biochemical markers[edit | edit source]
- there is no single reliable parameter of bone metabolism for the diagnosis of MBD, therefore a combination of several markers is used:
Recommendations for MBD screening in at-risk premature babies (all newborns with birth weight < 1.5 kg and also newborns with other risk factors):
MBD: P < 1.8 mmol/l, ALP > 8.3 μkat/l (> 500 IU/l), TRP > 95 % (→ phosphorus deficiency); parathyroid hormone > 180 mg/dL.[1]
A drop in the serum phosphorus level below 1.8 mmol/l, i.e. below the renal threshold for phosphorus, can be considered a sign of phosphate deficiency and a risk factor for the development of MBD in premature newborns. The test has good specificity (95%) but very low sensitivity (50%). Phosphorus deficiency may be the earliest sign of poor mineralization.
The serum calcium level is kept stable even at the cost of significant bone demineralization, so it cannot be used to diagnose MBD.
Serum alkaline phosphatase (ALP) level is a commonly used parameter of bone metabolism, although its usefulness is limited. In newborns, the bone isoform accounts for 90% of total ALP, the remainder being liver and kidney isoforms. By examining bilirubin and liver enzymes, the elevation of total (tissue non-specific) ALP can be distinguished from the elevation of the liver isoform (i.e. hepatocyte damage). Elevation of ALP accompanies normal bone growth, MBD healing and increased turnover in case of inadequate mineralization (in the absence of substrate for the formation of hydroxyapatite) - i.e. increased activity of osteoblasts and osteoclasts. In case of zinc deficiency or malnutrition (e.g. in newborns after intestinal resection requiring long-term parenteral nutrition), the ALP level may be unreasonably low. An association between ALP and vitamin D levels in very low birth weight infants was not demonstrated. No correlation between ALP level and X-ray signs of bone demineralization or bone density on densitometry was demonstrated. Therefore, ALP does not reflect the patient's mineral reserves, Ca and P supplementation cannot be controlled according to any of its values. Very high levels of ALP (> 20 μkat/l) are associated with small growth in childhood.[2][1]
ALP and P level combination ALP and P combination provides 100% sensitivity (ALP > 15 μkat/l and P < 1.5 mmol/l) but only 70% specificity for reduced bone density.[7]
Calcium and phosphorus wastes in the urine
To assess the adequate intake of minerals needed for the formation of hydroxyapatite, simple urinary concentrations of Ca and P or their creatinine indices, which take into account urine thickening, can be used. The disadvantage is the absence of clear reference limits for premature newborns. In extremely premature neonates, phosphorus excretion into the urine is increased even at low serum phosphorus levels.
Another option is to calculate tubular phosphate reabsorption (TRP) using the formula: 1- (U-P (mmol/l) x S-krea (mmol/l) / (U-krea (mmol/l) x S-P (mmol/l)) x 100. Tubular reabsorption above 95% is considered a sign of P deficiency in the body. This is a relatively complex calculation requiring knowledge of the serum creatinine value, does not provide any information about the state of calcium stores and may be affected by secondary hyperparathyroidism during Ca depletion (TRP will certainly be below 95% in parathyroid-induced phosphaturia).
Failed to parse (syntax error): {\displaystyle TRP=(1-\frac{P[U] × creatinine[S]}{P[S] × creatinine[U]}\,)\,×100}
Renal Ca load results from the difference between intestinal absorption and bone retention. The main factors influencing urinary Ca waste in premature newborns are phosphorus deficiency (not a substrate for the formation of hydroxyapatite → calciuria), insufficient intake/absorption of calcium and drugs (furosemide, corticosteroids, caffeine, aminophylline → calciuria despite a possible absolute deficiency). Phosphate absorption in the intestine is very efficient and unregulated. The kidneys have a key role in maintaining serum phosphorus levels through its excretion (regulated by parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23). Tubular reabsorption is an active and saturable process.
Phosphaturia occurs when the renal threshold for phosphorus is exceeded, thus the serum P level in VLBW newborns should be 1.8 mmol/l. When the serum drops below this value, all P from the urine is absorbed. The maximum serum P value above which reabsorption is 100% saturated is 2.45 mmol/L (further increases in renal load only increase urinary wastes). In extremely premature newborns (23-25 weeks of gestation), the renal threshold for phosphorus is even lower. Even with phosphateemia around 1 mmol/l, positive excretion of P was detected in the first 5 weeks of life, later the renal threshold rises to a serum value of 1.8 mmol/l.[2]
Vitamin D
Prenatally, the interaction of PTH and PTHrP (PTH related peptide) is necessary for normal development and optimal mineralization of the fetal skeleton, the presence of vitamin D (calcidiol, calcitriol) is not necessary. The postnatal role of activated vitamin D (calcitriol) is to ensure the absorption of Ca (and to a lesser extent P) from the intestine.
Hypovitaminosis D in premature children is among the risk factors for the development of metabolic bone disease in immaturity (MBD), leading to resorption of Ca from the bones through secondary hyperparathyroidism. The best marker to assess the status of vitamin D stores is 25-hydroxyvitamin D (25-OHD, calcidiol). Although vitamin D plays an important role in calcium-phosphate metabolism, determination of its serum level in the diagnosis of MBD is not part of the basic panel of investigations, as there are no clinical data to support it yet. It is advisable to monitor the serum level of vitamin D in newborns with a risk of malabsorption (cholestasis, bowel resection).[2]
The level of 25-OHD in the newborn correlates with the level of the mother during pregnancy - in full-term newborns, the level of 25-OHD is usually 70-100% of the level of the mother during pregnancy. During pregnancy, many women have vitamin D insufficiency or deficiency (25-OHD < 50 or 25 nmol/L). Hypovitaminosis D in a pregnant woman and in the umbilical cord blood of a newborn is associated with the development of type 1 diabetes mellitus in childhood, allergies, disorders of speech development, and impaired function of the newborn's immune system (at the level of the intestinal mucosa and respiratory tract mucosa).[8][9][10][11][12][2]
Parathyroid hormone
An increase in the level of parathyroid hormone (secondary hyperparathyroidism) is a sensitive indicator of calcium deficiency and could probably be used as an early marker of MBD after confirmation of the reference limits for premature newborns. Parathyroid hormone stimulates the proliferation and differentiation of osteoclasts and thus leads to further leaching of minerals from the already demineralized bone of immature newborns.[2]
| Vitamin D | Parathyroid hormone | Calcitonin | |
|---|---|---|---|
| kidneys | ↑ reabsorption of Ca2+ and phosphates | ↑ resorption of Ca2+and excretion of phosphates,
stimulates the production of calcitriol |
↑ excretion of Ca2+,
↑ excretion of phosphates |
| bone | bone mineralization;
high levels, on the other hand, decalcify |
bone resorption (osteoclast activation),
calcemia and phosphatemia rise |
inhibition of osteoclasts,
Ca2+ deposition in bones |
| intestine | stimulates resorption of Ca2+ and phosphates | stimulates the production of calcitriol
→ stimulates resorption of Ca2+ and phosphates |
– |
Other methods[edit | edit source]
An X-ray examination is used to objectify the state of the skeleton in the case of suspected MBD. However, it is not beneficial for the early diagnosis of MBD, and it is also burdened with subjective error. Typical changes can be detected with as little as 20-40% reduction in bone mineralization.
Bone densitometry measures bone density (the amount of minerals in the bone in the monitored section or bone volume). Two-photon X-ray absorptiometry (DEXA), which is currently considered the gold standard for the examination of adults, is used in neonatology only for clinical research.[2]
Quantitative ultrasound densitometry (QUS) - examination of the content of bone minerals and organic matrix is used in neonatology only for clinical research.[15]
Prevention and treatment[edit | edit source]
In premature babies, it is important to consistently supplement calcium, phosphorus, magnesium and vitamin D. Early initiation of enteral nutrition, shortening the duration of parenteral nutrition, fortification of breast milk (breast milk contains only about 0.5 mmol of phosphorus/100 ml)[5] or administration of special formula for premature babies, adequate supplementation of vitamin D. The table summarizes recommended daily doses and target serum values.
| Recommended daily intake for premature newborns[16] | |||
|---|---|---|---|
| Parenteral nutrition in the first days of life | parenteral nutrition after the first days of life | Enteral nutrition | |
| Calcium (Total) | 1,5 - 2 (min. 1) | 1,25 - 2 | 3 - 5,5 mmol/kg/day |
| Phosphorus | 1 - 2 | 1,25 - 3 (usually max 2 due to solubility) | 2,3 - 3,9 mmol/kg/day |
| Calcium: Phosphorus | 0,8-1 : 1 | 1,3-1,5 : 1 | 1,6-1,8 : 1 |
| Magnesium | 0,1 - 0,2 | 0,2 - 0,3 | 0,33 - 0,62 mmol/kg/day |
| Vitamin D | 400 | 400 | 400 - 1000 IU/day |
| Target serum levels[16] | ||
|---|---|---|
| Normal values | Deficit | |
| Calcium (Total) | 2,2 - 2,5 mmol/l | < 1,75 - 2,2 |
| Phosphorus | 1,8 - 2,6 mmol/l | < 1,3 |
| Magnesium | 0,7 - 1,5 mmol/l | < 0,7 |
| Vitamin D | 30 - 80 mmol/l | < 20 |
Other important factors:
- Physical activity or mechanical stimulation through passive exercise improves bone mineralization.
- Minimize the use of furosemide and corticosteroids.
- Increased attention in children with cholestasis and malabsorption (consistent supplementation of fat-soluble vitamins).[1]
Possibilities of supplementation[edit | edit source]
- Vitamin D - Vigantol;
- Ca and P - fortification of breast milk; calcium capsules; phosphate solution (Ca and P are given separately due to the risk of precipitation).
Sources[edit | edit source]
Related articles[edit | edit source]
External links[edit | edit source]
- Aplikace ke zhodnocení parametrů kostního metabolismu a doporučení terapie
- L. Petrů: Otrava fosfátovým sirupem
References[edit | edit source]
- ↑ a b c d e f GOMELLA, Tricia – EYAL, Fabien – BANY-MOHAMMED, Fayez. Gomella's Neonatology, Eighth Edition. 8. edition. McGraw-Hill Education, 2020. pp. 1022-1026. ISBN 9781259644818.
- ↑ a b c d e f g h i j k MATĚJEK, T. , et al. Metabolické kostní onemocnění při nezralosti. Čes-slov Pediat [online]. 2015, y. 70, vol. 5, p. 303-312, Available from <https://www.prolekare.cz/casopisy/cesko-slovenska-pediatrie/2015-5/metabolicke-kostni-onemocneni-pri-nezralosti-56516/download?hl=cs>.
- ↑ a b FAIENZA, Maria Felicia – D'AMATO, Elena – NATALE, Maria Pia. Metabolic Bone Disease of Prematurity: Diagnosis and Management. Frontiers in Pediatrics. 2019, y. ?, vol. 7, p. ?, ISSN 2296-2360. DOI: 10.3389/fped.2019.00143.
- ↑ KOVACS, Christopher S.. Bone Development in the Fetus and Neonate: Role of the Calciotropic Hormones. Current Osteoporosis Reports. 2011, y. 4, vol. 9, p. 274-283, ISSN 1544-1873. DOI: 10.1007/s11914-011-0073-0.
- ↑ a b c d RENNIE, JM, et al. Textbook of Neonatology. 5. edition. Churchill Livingstone Elsevier, 2012. pp. 920. ISBN 978-0-7020-3479-4.
- ↑ ABRAMS, Steven A. – BHATIA, Jatinder J. S. – ABRAMS, Steven A.. Calcium and Vitamin D Requirements of Enterally Fed Preterm Infants. Pediatrics. 2013, y. 5, vol. 131, p. e1676-e1683, ISSN 0031-4005. DOI: 10.1542/peds.2013-0420.
- ↑ Backström MC, Kouri T, Kuusela AL, et al. Bone isoenzyme of serum alkaline phosphatase and serum inorganic phosphate in metabolic bone disease of prematurity. Acta Paediatr 2000; 89 (7): 867–873
- ↑ Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child 2008; 93 (6): 512–517.
- ↑ Reinholz M, Ruzicka T, Schauber J. Vitamin D and its role in allergic disease. Clin Exp Allergy 2012; 42 (6): 817–826.
- ↑ Whitehouse AJ, Holt BJ, Serralha M, et al. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics 2012; 129 (3): 485–493.
- ↑ Walker VP, Zhang X, Rastegar I, et al. Cord blood vitamin D status impacts innate immune responses. J Clin Endocrinol Metab 2011; 96 (6):1835–1843.
- ↑ Dinlen N, Zenciroglu A, Beken S, et al. Association of vitamin D deficiency with acute lower respiratory tract infections in newborns. J Matern Fetal Neonatal Med 2015; 19: 1–5.
- ↑ SILBERNAGL, Stefan – DESPOPOULOS, Agamemnon. Atlas fyziologie člověka : 6. vydání, zcela přepracované a rozšířené. 3. edition. Grada, 2004. pp. 290-293. ISBN 80-247-0630-X.
- ↑ LEBL, J – JANDA, J – POHUNEK, P, et al. Klinická pediatrie. 1. edition. Galén, 2012. pp. 189-196. ISBN 978-80-7262-772-1.
- ↑ KORČEKOVÁ, Zuzana – KORČEK, Peter – ČUNÁT, Václav. Tibial speed of sound changes in preterm infants during the first year of life. Bone. 2020, y. ?, vol. 132, p. 115191, ISSN 8756-3282. DOI: 10.1016/j.bone.2019.115191.
- ↑ a b KOLETZKO, Berthold. Nutritional Care of Preterm Infants : Scientific Basis and Practical Guidelines. - edition. Karger, 2021. pp. 138, 147. ISBN 9783318066463.