Flow cytometry
Flow cytometry (flow cytometry; the combination flow+cyto+metry can be loosely understood as measurement of cells in motion) is a demanding, yet rapidly developing, instrumental method used both in clinical practice , both in research laboratories. Its great advantage is the fact that it is possible to analyze the properties at the cell level in a sample with a large number of cells in a very short period of time. FACS (Fluorescent Activated Cell Sorting) is a specialized type of flow cytometry. It provides a method for sorting individual cells of a heterogeneous mixture of biological cells into two or more containers, based on the specific light scattering and fluorescence properties of each cell.
Main parts of a flow cytometer[edit | edit source]
A flow cytometer consists of three main parts: fluidics, optics and electronics.
Fluidics[edit | edit source]
For the analysis, it is necessary that the cells are in the form of a suspension (it is necessary that the particles pass through the system one after the other and that they pass at a constant speed). Blood or cultured cell samples are easily dispersed in the appropriate solution, but cell suspensions can also be made from fixed and embedded tissue samples. Although the flow cytometer was originally built for the analysis of cells, today it can be used for the analysis of smaller particles (meaning suspensions of these particles), for example viruses and chromosomes. But a lack of cells can be a problem. The analyzed suspension should preferably contain 105–106 particles in one milliliter. The optimal particle diameter should be 1–30 µm. [1]
[2]
A suspension of labeled cells is dispensed into a test tube. It comes out of the test tube at the lower end of the capillary. The test tube is placed in a container with a jacket liquid, which flows away in laminar flow and drags individual cells with it from the capillary. In order for just one particle to pass through the measuring point, it is ensured by 'hydrodynamic focusing. In the case of a large pressure difference between the sample and the carrier fluid (at a high sample pressure), for example, at a pressure difference of 5.5 kPa, the sample stream is wide, so more cells flow through the surface cross-section. With a large pressure difference, the resolution is worse. In the case of a small pressure difference between the sample and the carrier fluid (at a low sample pressure), for example at a pressure difference of 1.3 kPa, the sample stream is narrow and therefore only a single cell will pass in the area cross-section. This method provides better resolution. For example, a small pressure difference is suitable for DNA analysis.
Furthermore, it is necessary to ensure that the flow is laminar and not turbulent. Whether the flow is laminar or turbulent can be determined by Reynolds number Re. The critical value of the Reynolds number Re is just the boundary between laminar and turbulent flow. This value is directly proportional to the tube diameter d, the fluid density ρ and the mean flow velocity v, and inversely proportional to the viscosity of the fluid η. [3]
Fluorochrome labeling[edit | edit source]
In the analyzed cells, the detected substance (for example, the product of cellular synthesis by immunohistochemistry, DNA Feulgen reaction) is represented by a suitable method so that it can be recognized by the device's detection system. Although some cells are capable of internal fluorescence due to the presence of internal fluorophores (for example cytochrome, peroxidase, hemoglobin), in most cases very specific fluorochromes are used. For fluorochromes, it is advisable that their absorption maximum is as close as possible to the wavelengths of commonly used lasers (for an overview of the absorption and emission spectra of fluorochromes, see [1] ). This connection explains why for many fluorochromes the excitation wavelength is λ= 488 nm (or a wavelength very close to it) and why an argon laser that emits radiation of wavelength λ= 488 nm is also used. A Stokes shift is defined for each fluorochrome. The Stokes shift is the difference between the excitation wavelength and the emission wavelength (emission radiation has less energy, therefore less frequency, therefore greater wavelength than excitation radiation). For an example of the excitation spectrum, emission spectrum and Stokes shift for the fluorochrome FITC (fluorescein isiothiocyanate), see the manual diagram.
The table shows the most frequently used fluorochromes in flow cytometry. [4]
| abbreviation | name | excitation wavelength (nm) | emission wavelength (nm) |
|---|---|---|---|
| FITC | Fluoresceinisothiocyanate | 494 | 520 |
| APC | Allophycocyanin | 630 | 661 |
| PE | Phycoerythrin | 488 | 575 |
| PerCP | Peridinin-chlorophyl-protein complex | 488 | 675 |
| PE-Cy5 | Phycoerythrin-Cyanin 5 | 488 | 670 |
| PE-Cy7 | Phycoerythrin-Cyanin 7 | 488 | 774 |
| APC-Cy5.5 | Allophycocyanin-Cyanin 5.5 | 633 | 695 |
| APC-Cy7 | Allophycocyanin-Cyanin 7 | 633 | 785 |
| PE-Texas Red | Phycoerythrin-Texas Red | 488 | 615 |
| PerCP-Cy5.5 | Peridinin-chlorophyl-protein complex-Cyanin 5.5 | 482 | 690 |
| - | AlexaFluor 488 | 495 | 519 |
| - | AlexaFluor 647 | 650 | 668 |
| - | AlexaFluor 594 | 590 | 617 |
| - | AlexaFluor 700 | 696 | 719 |
| - | Pacific Blue | 410 | 455 |
| - | Pacific Orange | 410 | 551 |
| eGFP | Enhanced Green Fluorescent Protein | 498 | 508 |
Optics[edit | edit source]
Lasers[edit | edit source]
The sample is irradiated with monochromatic radiation, lasers are most commonly used. Flow cytometers are usually two-laser or three-laser. The most commonly used laser is an air-cooled argon laser that emits radiation with a wavelength of 488 nm (blue region of the spectrum), a helium-neon laser with a wavelength of 633 nm (red region of the spectrum ) and a violet laser with a wavelength of 407 nm or a UV laser with a wavelength of 350 nm. [5]
Filters[edit | edit source]
The collection optics consists of a system of filters, mirrors and lens. Mirrors and filters divide the emitted photons according to wavelength to the appropriate detectors (photons are divided into so-called channels). As for filters, long pass filters, short pass filters, bandpass filters and dichroic filters are used.
Short pass filters (SP) pass all wavelengths lower than a specific wavelength (e.g. 600SP passes all wavelengths shorter than λ=600 nm).
Long pass filters (LP) pass all wavelengths higher than a specific wavelength (e.g. 600LP passes all wavelengths longer than λ=600 nm).
Band filters pass a specific range of wavelengths. For example, the 575/25BP filter used for the fluorochrome PE (Phycoerythrin) passes the wavelength band 562.5 nm to 587.5 nm (ie 575 ± 12.5 nm; figure for therefore, the slash does not represent ± 25 nm, but the range of transmitted wavelengths in total, i.e. the difference between the longest and shortest wavelength that it transmits !!!) and the 780/60BP filter used for PE-Cy7 transmits the band 750 nm to 810 nm. The use of specific filters is related to the emission wavelength of each fluorochrome. The 575/25BP filter correlates with the emission wavelength of PE (λemission =575 nm) and the 780/60BP filter correlates with the emission wavelength of PE-Cy7 (λemission =774 nm ). See page 19 for an overview of the filters used link
With diachroic filters that are placed at an angle of 45°, some light passes through and some is reflected.

FSC and SSC[edit | edit source]
The detectors record:
- forward scatter (FSC)
- side scatter (SSC)
- fluorescence
In the case of side scatter (SSC) and fluorescence, the signal intensity is low and therefore the signal needs to be amplified by a photomultiplier. With direct scattering (FSC) the intensity is sufficient, it is not necessary to amplify it. The intensity of the FSC rays is directly proportional to the size of the cell, the intensity of the SSC rays reflects the internal complexity of the cells and is proportional to the granularity of the cell (state of the cytosol, cell inclusions, granules, etc.). Granulocytess and apoptotic cells have higher SSC values, which are probably caused by nucleus granulation or fragmentation. According to the intensity of FSC rays, it is possible to distinguish between living and dead cells, and according to SSC, granulous and agranulous cells.[6]
Electronics (analysis and interpretation of acquired data)[edit | edit source]
The signals from the optics are converted into electrical impulses that are processed by a computer program.
During processing, you must:
- remove so-called conflicting cases (two cells were measured at the same time)
- combine data measured separately in time when it is a signal of the same cell
- to compensate for the glow of fluorochromes (compensate for overlapping emission spectra of fluorochromes).
The result is then expressed graphically in the form of either a single-parameter histogram (signal intensity is displayed on the x-axis, frequency on the y-axis) or two-parameter using dot plots.
In one graph, it is therefore possible to observe forward scattering (FSC), side scattering (SSC) and fluorescence (the fluorescence signal is provided by those particles that have a bound antibody with a fluorochrome). A population of cells is selected from the graph (so-called gating), which will be the subject of further analysis, and a dot plot histogram is created for it, where the parameters to be investigated are plotted on both axes.
[1]
[5]
Illustrative example[edit | edit source]
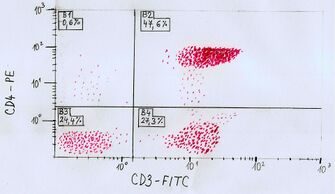
The illustrative example (below) refers to a picture (a drawing made by my own hand according to the link [2]
, image from the ninth page of the presentation).
One of the parameters is plotted on the x-axis. In this case, the presence of the epitope of CD3, or the fluorochrome FITC (Fluoresceinisothiocyanate), which is bound to a monoclonal antibody against the CD3 epitope (antigen). CD3 epitope (CD=Clusters of Differentiation, so-called differentiation features, features of a defined structure recognizable by a monoclonal antibody, currently more than 200 leukocytes have been characterized and marked on human leukocytes [antibody|antibody]]) [7] [8]
is present on the surface of lymphocytes.
The second parameter is plotted on the y-axis. In this case, it is a CD4 epitope, or the binding of PE (Phycoerythrin) fluorochrome to a monoclonal antibody against CD4 epitope is recorded here. The CD4 epitope is found in a subpopulation of helper lymphocytes.
The graph is divided into 4 quadrants.
- In the 1st quadrant, cells are positive only for the sign plotted on the y-axis, i.e. in this particular case cells positive for antigen CD4 (0.6% of cells in this illustrative example).
- In the 2nd quadrant, cells are positive for signs plotted on both axes (x,y), i.e. the cells of this quadrant contain both antigeny (CD3 and CD4). In this illustrative example, approximately 47.6% show positivity for both antigens.
- The cells of the 3rd quadrant are negative for both monitored signs. In the illustrative example, therefore, 24.4% of the cells are located in this quadrant.
- In the 4th quadrant, cells are positive only for the sign plotted on the x-axis, in this case only for antigen CD3. About 27.3% of the cells are located in the fourth quadrant.
Relative intensity of fluorescence is plotted on the axes in an exponential scale.
Table of occurrence of epitopes (antigenic determinants) investigated in flow cytometry[9] [10] [1]
| epitopes | occurrence |
|---|---|
| CD38+ | plasma cells |
| CD14+ | monocytes |
| CD15+ | granulocytes |
| CD3+ | mature T lymphocytes |
| CD4+ | a subpopulation of helper T lymphocytes |
| CD8+ | a subpopulation of cytotoxic T lymphocytes |
| CD25+ | activated T lymphocytes |
| CD19+, CD5+ | mature B lymphocytes |
| CD56+, CD16+ | NK cells |
Types of sorters[edit | edit source]
According to the method of work, we distinguish two basic groups of sorters:
- electrostatic droplet sorter
- fluidic switch sorter (fluidic switch sorter)
Electrostatic droplet sorter[edit | edit source]
It uses electrostatic forces to deflect cells. The cells are injected into the air in a stream of carrier liquid, vibration waves are used to break the continuous stream of liquid into droplets. Charged droplets are deflected from the main stream towards one of the charged plates, these droplets are collected while the uncharged main stream goes to the waste tank. The droplet sorter has a high separation speed and the separated populations are concentrated.
Fluid switch sorter[edit | edit source]
The fluid switch sorter enables the sorting of even large particles and does not pose a biological risk, but there is greater dilution of the separated population and the sorting speed is also lower than with the droplet sorter. These sorters can work on different principles. In the first principle, the stream of the carrier liquid with the sample passes through the channel, when a particle meeting the required criteria is identified, the piezoelectric valve is closed and the stream with the particle deviates from the main stream. In the second principle, the collection tube for the desired sample is moved to the center of the stream flow and is extended and retracted into the liquid stream in case of detection of the desired particle.
Sort speed[edit | edit source]
The length of the sort depends on the quality of the sample and the type of cells. The sort of small cells (e.g. thymocytes) takes less time than the sort of large, fragile, adherent cells. Under optimal conditions, 1 million macrophages are sorted in 3 minutes, 1 million thymocytes are sorted in about 45 seconds. For example, one flow cytometer with high-speed sort is stated to have a standard sorting speed of 6,000 cells/second and to be able to separate up to 25,000 cells per second in high-speed mode. [11]
Advantages and Disadvantages of Flow Cytometry[edit | edit source]
Benefits[edit | edit source]
- The advantage of this technique lies primarily in the high frequency of analysis of individual particles.
- Large amount of analyzed material - large volumes of data.
- The analysis only takes a few minutes and provides a large amount of information.
- Both qualitative and quantitative analysis can be performed.
- Manipulation operations are possible here - e.g. sorting cells with selected properties (cell sorting). Sorting is primarily intended for scientific purposes, but is not used much in clinical practice.
- Simple sample preparation for analysis. (The whole preparation usually takes no more than 1 hour.)
Disadvantages[edit | edit source]
- The disadvantage of flow cytometry is its high financial cost - the limiting factor for practical use is the price of flow cytometers.
- Experiment setup, data analysis and evaluation depends on the experience of the operator.
- When analyzing solid tissues, it is necessary to separate individual cells from each other. During this separation, however, cell properties may change or be lost.
Utilization of flow cytometry[edit | edit source]
- Research on plant, animal and human cells.
- Yeast research as an increasingly important model of higher eukaryote.
- Applications of flow cytometry reach all branches of clinical practice.
They are most widely used in clinical immunology, hemato-oncology, tumor biology and in the field of molecular biology.
Flow cytometry is mostly used to examine:
- BLOOD - in absolute and relative representation - total T lymphocytes, helper/inducer T lymphocytes, suppressor/cytotoxic T lymphocytes, activated T lymphocytes, B lymphocytes, activated/immature B lymphocytes, NK cells spontaneous cytotoxicity, examination of platelets - reticulated platelets, activated platelets, platelet glycoproteins, bound immunoglobulins on platelets; illustrative example request forms for examination of peripheral blood by flow cytometry
- BLOOD MARROW - similar indication as for examination of peripheral blood; a special test tube with anticoagulant solution is used for sampling
- LYMPHATIC NODES - for example, diagnosis of lymphomas, differentiating lymph node cancer from a reactive node; removal into gauze moistened in saline solution
- CEREBROSPINAL FLUID (cerebrospinal fluid)
- OUTLETS
- BRONCHOALVEOLAR LAVAGE (BAL)
Flow cytometry is therefore used, for example, to distinguish between reactive and monoclonal lymphocytosis, to differentiate between acute lymphoblastic and myeloid leukemia, to rule out or confirm paroxysmal nocturnal hemoglobinuria and HCL hairy cell leukemia in pancytopenia, to diagnose lymphomas, thrombocythemia , in the diagnosis of immune defects (for example, the diagnosis and monitoring of autoimmune diseases), in transplantation immunology and much more. [9]
[12]
[13]
[14]
Flow cytometry is also used for
- DNA and RNA analysis
- testing activation of basophils to a specific allergen or group of allergens (allergy to weeds - e.g. ragweed, wormwood; grasses; woody plants; mites, fungi; to animals - to dogs, for cats; for insects – wasps, bees) [15]
- examination of acrosome integrity - the ability of sperm to penetrate the oocyte. [16]
- lifetime analysis (representation of apoptotic cells)
- measurement of the concentration of intracellular calcium (measurements with the help of a fluorescence microscope can also be used for this determination). [17]
Reimbursement from health insurance[edit | edit source]
Flow cytometry is a procedure covered by health insurance companies. In the List of medical procedures with point values, issued by the Ministry of Health of the Czech Republic for the year 2013, it is under the procedure code 91439 - Immunophenotyping of cell subpopulations according to surface features - flow cytometry and 91475 - Written interpretation of a set of immunological laboratory examinations by a laboratory worker - a doctor specialist in the field of medical immunology. Performance 91439 is rated 319 points and performance 91475 is rated 116 points. Both services can only be contracted at a specialized workplace.
Number of performances reported[edit | edit source]
In the first half of 2013, the General Health Insurance Company of the Czech Republic paid for 42,028 flow cytometry procedures (procedure code 91439) for its clients throughout the Czech Republic. [18]
References[edit | edit source]
Links[edit | edit source]
- overview of emission and absorption spectra of the most used fluorochromes http://www.bdbiosciences.com/us/applications/s/spectrumguidepage
- overview of filters used for individual fluorochromes in flow cytometry (page 19 of the link) http://biomedicina.prf.jcu.cz/wordpress/wp-content/uploads/2011/12/Vyuzitiprutokovecytometrievimunologii.pdf
- original source of the image used in the illustrative example (from the ninth page of the presentation) http://web.lfp.cuni.cz/imunologie/Praktika/2.c%20seminar%20Prutokova%20cytometrie.ppt
- example of a requisition form for a flow cytometry examination http://www.uhkt.cz/zdravotnik/komplement-laboratori/laboratorni-prirucky-zadanky/laborator-prutokove-cytometrie/116_LP_08_01_Priloha1.pdf
- ↑ a b c ROUBALOVÁ, L.. Průtoková cytometrie [online]. [cit. 2013-25-11]. <http://web2.stapro.cz/bullfons/22012/labo1.pdf>.
- ↑ SHAPIRO, Howard M. Practical flow cytometry 4th ed. 4.vydání edition. John Wiley and sons, 2003. ISBN 978-0-471-41125-3.
- ↑ NAVRÁTIL, Leoš – ROSINA, Jozef. Medicínská biofyzika. 1 (dotisk 2013) edition. Grada Publishing, 2005 (dotisk 2013). 524 pp. ISBN 978-80-247-1152-2.
- ↑ LANGHANSOVÁ, HELENA. Využití průtokové cytometrie v imunologii [online]. České Budějovice - Jihočeská univerzita, 2011, Available from <http://biomedicina.prf.jcu.cz/wordpress/wp-content/uploads/2011/12/Vyuzitiprutokovecytometrievimunologii.pdf>.
- ↑ a b teaching presentation of the 2nd Medical Faculty of the Charles University in Prague, Průtoková cytometry, Prague 2009, http://immunologie.lf2.cuni.cz/soubory_vyuka/Prutokova%20cytometrie_Laboratorni%20vysetrovaci%20metody.pdf
- ↑ BERNAND, Vladan. Průtoková cytometrie : flow-cytometrie [online]. Brno - Biofyzikální ústav Lf Masarykovy univerzity, -, Available from <http://www.med.muni.cz/biofyz/files/gerontologie/prutokova_cytometrie_prezentace.pdf>.
- ↑ Laboratoř AIDS a infekční imunologie. Princip průtokové cytometrie [online]. [cit. 2013-12-01]. <http://www1.lf1.cuni.cz/~hrozs/flowcyt1.htm>.
- ↑ Oddělení fyziologie a imunologie živočichů PřF Masarykovy univerzity. Práce s buňkami imunitního systému [online]. Brno, -, Available from <http://www.sci.muni.cz/ofiz/index_cz.php?page=studies&sub=materialy>.
- ↑ a b laboratorní vyšetření v klinické imunologii a alergologii. přehled vyšetření -> Stanovení subpopulací lymfocytů periferní krve [online]. [cit. 2013-11-29]. <http://www.interimun.cz/prehled-vysetreni-13-stanoveni-subpopulaci-lymfocytu-periferni-krve.htm/>.
- ↑ Lékařská fakulta UK v Plzni. Průtoková cytometrie (2.c seminar prutokova cytometrie.ppt). Plzeň, -,
- ↑ {{Mikrobiologický ústav Akademie věd ČR. FACS Vantage SE [online]. [cit. 2013-12-01]. <http://www.cytometry.cz/sysmenu.php?rstext=odkaz&rstema=17&rslink=sysmenu/VantageCZ.htm&stromhlmenu=9:17>.
- ↑ BRZOVÁ, Petra. Průtoková cytometrie základní princip a klinické využití. Nemocnice s poliklinikou Havířov, -,
- ↑ ÚHKT - Ústav hematologie a krevní transfuze. žádanka o vyšetření průtokovou cytometrií [online]. [cit. 2013-11-30]. <http://www.uhkt.cz/zdravotnik/komplement-laboratori/laboratorni-prirucky-zadanky/laborator-prutokove-cytometrie/116_LP_08_01_Priloha1.pdf>.
- ↑ Ústav alergologie a imunologie FN Plzeň. Vyšetření parametrů buněčné imunity : název souboru Stanoveni parametru bunecne imunity.ppt. Plzeň, -,
- ↑ imalab. test aktivace bazofilů [online]. [cit. 2013-11-30]. <http://www.imalab.cz/clanek/266-test-aktivace-bazofilu.aspx>.
- ↑ imalab. integrita akrozomu [online]. [cit. 2013-11-30]. <http://www.imalab.cz/clanek/262-integrita-akrozomu.aspx>.
- ↑ ČÍHAL, Martin. METODY MĚŘENÍ KONCENTRACE INTRACELULÁRNÍHO VÁPNÍKU [online]. Brno : Vysoké učení technické v Brně, 2011, Available from <https://dspace.vutbr.cz/bitstream/handle/11012/2001/Bakal%C3%A1%C5%99sk%C3%A1%20pr%C3%A1ce-xcihal04.pdf?sequence=1>.
- ↑ source: Information system of VZP ČR
Source[edit | edit source]
- JIRKOVSKÁ, Marie. Histologická technika : Pro studenty lékařství a zdravotnické techniky. 1. (dotisk 1. vydání) edition. Galén, 2009. ISBN 978-80-7262-263-4.
- Přírodovědecká fakulta Masarykovy univerzity. Genotoxicita a karcinogeneze - oddíl kontrola hemopoézy a leukemie - pododdíl Diagnostika, léčba, terapeutické využití cytokinů [online]. [cit. 2013-12-01]. <https://is.muni.cz/do/rect/el/estud/prif/ps13/genotox/web/pages/12_kontrola.html>.
- NOVÁK, BASAŘOVÁ, FIALA, DOSTÁLEK, Jan, Gabriela, Jaromír, Pavel. Průtoková cytometrie ve výzkumu kvasinek Saccharomyces cerevisiae a její aplikace v praxi [online]. [cit. 2013-25-11]. <http://www.chemicke-listy.cz/docs/full/2008_03_183-187.pdf>.
Recommended literature[edit | edit source]
- PROF. MUDR. ECKSCHLAGER, Tomáš, Csc.. Průtoková cytometrie v klinické praxi. 1.vydání edition. Grada Publishing, 1999. ISBN 80-7169-279-4.