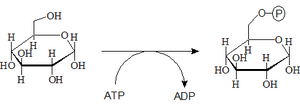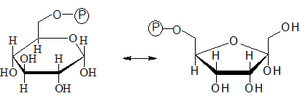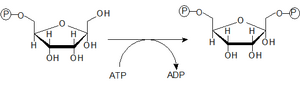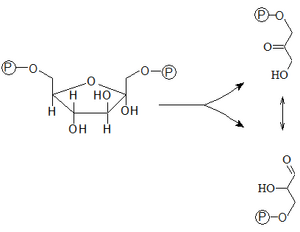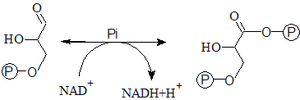Embden-Meyerhof-Parnassus pathway
Glycolysis (or the Embden-Meyerhof-Parnas pathway) is a basic metabolic process taking place in the cytoplasm of all cells of the human body. It ranks among catabolic pathways. Glycolysis produces two three-carbon molecules from one glucose molecule - pyruvate (Pyr) or lactate (Lac). Glycolysis fulfills many functions, for example the gain of energy or the formation of acetyl-CoA as a substrate for the synthesis of lipids.
Glycolysis takes place under both aerobic and anaerobic conditions. Under aerobic conditions, two molecules of pyruvate, two molecules of ATP and two molecules of NADH are produced. Under anaerobic conditions, pyruvate undergoes another reaction that regenerates the cofactor NAD+ - the product is then lactate.
Glycolysis reaction[edit | edit source]
The entire glycolysis can be summarized as an equation:
- Glucose + 2 NAD+ + 2 ADP + 2 Pi → 2 pyruvate + 2 NADH + 2 ATP + 2 H2 O
Glycolysis is divided into several phases:
- Investment of energy and simultaneous activation of glucose molecules.
- Cleavage of a hexose into two trioses.
- Oxidation of triose and simultaneous energy gain.
- Conversion of pyruvate to lactate (under anaerobic conditions).
- In the following overview, we will describe her individual reactions
- 1. Phosphorylation of glucose
- After glucose molecules enter the cells, their immediate phosphorylation occurs. This reaction converts the neutral glucose molecule into an anion. Glucose modified in this way can be further metabolized and at the same time no longer "passes through the cell membrane". It is thus captured in the cytosol, where it is further metabolized.
- Glucose + ATP → Glc-6-P + ADP
- In addition to one macroergic bond of the ATP molecule, the reaction also requires enzyme catalysis mediated by one of the two isozymes - "hexokinase" or "glucokinase".
- Glucokinase (or hexokinase type IV) is localized only in hepatocytes and pancreatic β-cells, while hexokinase is in all tissues. In addition to localization, they also differ in their physico-chemical properties. Glucokinase has a high KM value (10 mmol/l) and is therefore only activated at higher glucose concentrations. It is mainly used after a meal, when the concentration of glucose in the portal blood is high and it needs to be taken up by the liver (e.g. for glycogen synthesis). At the same time, β-cells of the pancreas respond to higher blood glucose levels by increasing insulin secretion.
- Hexokinase is always almost fully active under physiological conditions, as its KM is only 0.1 mmol/l (compare with the physiological range of glycemia 3, 3–5.6 mmol/l). Its activity is thus regulated by a different mechanism, and that is the inhibition by its own product – Glc-6-P. Simply put, hexokinase produces as much Glc-6-P as the cell is able to utilize in its pathways. Once Glc-6-P starts to accumulate, hexokinase inhibition occurs. In addition to the phosphorylation of glucose, hexokinase also enables the phosphorylation of fructose.
- 2. Isomerization of Glc-6-P to Fru-6-P
- Isomerization of Glc-6-P to Fru-6-P is a reversible reaction catalyzed by hexosaphosphate isomerase.
- 3. Phosphorylation of Fru-6-P under ATP consumption to Fru-1,6-bisP
- Phosphorylation of Fru-6-P to Fru-1,6-bisP is catalyzed by the enzyme 6-phosphofructo-1-kinase. It is a key allosteric regulatory enzyme of glycolysis.
- During the three steps so far, two molecules of ATP have been invested for one molecule of glucose.
- 4. Aldol cleavage of Fru-1,6-bisP into two phosphorylated trioses
- Fru-1,6-bisP is subsequently split into two phosphorylated trioses – glyceraldehyde-3-P (Gra-3-P, aldose) and dihydroxyacetone-3-P (DHA-3-P, ketose). Catalysis is provided by aldolase belonging to the class of lyases. We distinguish between its two isozymes - aldolase A and B.
- 5. Isomerization of trioses
- Glyceraldehyde-3-P and dihydroxyacetone-3-P can be converted into each other by the enzyme ``triose phosphate isomerase. This reaction is of great importance, because only glyceraldehyde-3-P is involved in the next reaction of glycolysis, and this isomerization continuously replenishes its cytosolic pool.
- 6. Oxidation of glyceraldehyde-3-P to 1,3-bisphosphoglycerate
- This reaction is the only oxidation reaction in all of glycolysis. The oxidation is catalyzed by ``glyceraldehyde-3-phosphate dehydrogenase. The reaction produces 1,3-bisphosphoglycerate (an energy-rich compound) and a reduced cofactor – NADH+H+. The reaction is exergonic – Pi binds to the newly formed COO– group by oxidation via a macroergic anhydride bond.
- 7. Conversion of 1,3-bisphosphoglycerate to 3-phosphoglycerate
- 1,3-bisphosphoglycerate is hydrolyzed to 3-phosphoglycerate by phosphoglycerate kinase. At the same time, substrate phosphorylation will occur (phosphorylation at the substrate level) - ATP is formed from ADP.
- 8. Isomerization of 3-phosphoglycerate to 2-phosphoglycerate
- Isomerization is catalyzed by phosphoglycerate mutase.
- 9. Dehydration of 2-phosphoglycerate to phosphoenolpyruvate (PEP)
- Dehydration of 2-phosphoglycerate is catalyzed by the enzyme enolase. The reaction leads to the formation of the macroergic compound phosphoenolpyruvate, which contains an ester-bound phosphate group.
- 10. Conversion of phosphoenolpyruvate to pyruvate
- Pi cleavage takes place first, then the unstable enol-pyruvate isomerizes to the more stable keto-pyruvate. A large amount of free energy is released during this transformation. This reaction is therefore strongly exergonic and practically irreversible. The released energy is used to synthesize ATP from ADP – substrate phosphorylation.
- The reaction is catalyzed by the regulatory enzyme pyruvate kinase.
During the 4th-10th reactions produced two molecules of ATP per one three-carbon fragment (Pyr). The energy balance of the entire glycolysis is thus +2 moles of ATP per 1 mole of glucose (−2 ATP consumed, +4 ATP created).
Metabolic fates of pyruvate[edit | edit source]
Pyruvate is the branch point of glycolysis. The fate of pyruvate depends on the oxidation state of the cell – NADH must be reoxidized to NAD+.
Under aerobic conditions, pyruvate is transported to the matrix of the mitochondria, where it is converted to acetyl-CoA via the pyruvate dehydrogenase reaction, which can participate, for example, in the Krebs cycle. The reduced cofactor NADH cannot simply transfer to the matrix mitochondria, where it should be involved in processes in the respiratory chain, because the mitochondrial membrane is impermeable to it. That is why it is used to reduce some substances – for example, cytoplasmic oxaloacetate to malate or dihydroxyacetone-P to glycerol-3-P. The resulting products already pass through the inner mitochondrial membrane and thus transport reducing equivalents into the mitochondrion. We are talking about the so-called shuttle mechanism or simply shuttles. For the transfer of NADH, there are two different shuttles in the cell - glycerol-phosphate and malate-aspartate. In the mitochondrion, the above reactions take place in the opposite direction:
- Malate + NAD+ → oxaloacetate + NADH+H+
- Glycerol-3-P + FAD → dihydroxyacetone-P + FADH2
The obtained reduced cofactors can subsequently enter the mitochondrial respiratory chain, where they are regenerated - ATP is simultaneously produced by aerobic phosphorylation. The return of oxaloacetate (OAA) back to the cytosol is not direct. It first requires a transamination to aspartate' catalyzed by aspartate aminotransferase (AST). The opposite reaction takes place in the cytosol - OAA is formed.
Under anaerobic conditions (e.g. an intensively working muscle with insufficient oxygen supply) or in erythrocytes, pyruvate is transformed into lactate, which is subsequently released from the cell into the bloodstream. At the same time, NAD+ regeneration occurs. The reaction is catalyzed by the enzyme lactate dehydrogenase (LDH):
- Pyruvate + NADH + H+ → lactate + NAD+
The NAD+ created by this reaction is a coenzyme for glyceraldehyde-3-phosphate dehydrogenase, without which glycolysis would stop. The resulting lactate can either participate in the Cori cycle or be oxidized in tissues with aerobic metabolism (heart, liver) to CO2 and H2O. Accumulation of lactate causes a drop in pH, which causes muscle pain and fatigue.
At this point it should be recalled that aerobic glycolysis produces much more ATP per 1 mole of glucose than anaerobic glycolysis.
2,3-BPG shunt[edit | edit source]
In erythrocytes, a branch of glycolysis called the 2,3-BPG shunt plays an important role. 1,3-bisphosphoglycerate is converted to 2,3-bisphosphoglycerate. This intermediate product no longer contains macroergically bound phosphate, and during its further conversion to 3-phosphoglycerate, no ATP is synthesized – only inorganic phosphate is released. The erythrocyte thus obtains less ATP during this course of glycolysis. But the significance of the detour lies in the fact that 2,3-bisphosphoglycerate reduces the affinity of hemoglobin to oxygen, i.e. it participates in the regulation of oxygen transport to hemoglobin.
Regulation of glycolysis[edit | edit source]
The regulatory points in glycolysis are three enzymes:
- 6-phosphofructo-1-kinase;
- pyruvate kinase;
- hexokinase.
- These enzymes catalyze irreversible exergonic reactions.
6-phosphofructo-1-kinase (PFK-1)[edit | edit source]
Phosphofructokinase, an allosteric enzyme regulated by several activators and inhibitors, is the main regulatory point of glycolysis:
- Increasing the ATP / AMP ratio leads to inhibition of glycolysis
- Glycolysis is a process leading to the production of ATP.
- ATP is a substrate and at the same time an allosteric inhibitor of this enzyme. AMP, on the other hand, acts as an enzyme activator. With an excess of ATP, further consumption of glucose as a nutrient stops.
- Citrate inhibits glycolysis
- When fatty acids are oxidized, the resulting acetyl-CoA inhibits PDH.
- The resulting pyruvate goes into carboxylation to oxaloacetate. If there is enough acetyl-CoA and oxaloacetate at the same time, citrate is synthesized, which accumulates in front of the enzyme isocitrate dehydrogenase. Citrate escapes into the cytoplasm where it blocks the regulatory enzyme of glycolysis. It signals that there are enough substrates of the Krebs cycle in the mitochondrion, and therefore there is no need to create more.
- Fructose-2,6-bisphosphate (Fru-2,6-P)
- Fructose-2,6-bisphosphate, an activator of glycolysis, acts as an extended arm of insulin - its concentration increases if the insulin / glucagon ratio is increased. It arises from fructose-6-P by a reaction catalyzed by 6-phosphofructokinase-2 (PFK-2).
- Glycolysis is activated by insulin and inhibited by counterregulatory hormones
- Increased insulin/glucagon ratio decreases intracellular cAMP concentration; this results in a predominance of dephosphorylation events. A decrease in the ratio and the action of other counterregulatory hormones will, on the contrary, cause an increase in the concentration of cAMP - phosphorylation events prevail. 6-phosphofructo-1-kinase is active in the dephosphorylated form.
- Acid pH inhibition
- 6-phosphofructo-1-kinase is inhibited by protons. Both pyruvate and lactate are relatively strong acids and their significant accumulation could endanger the cell. Therefore, their increased concentrations lead to the inhibition of the regulatory enzyme through protons.
The remaining enzymes are of minor importance, so we will only describe them very briefly. As we have already mentioned, hexokinase is inhibited by its product – Glc-6-P, and activated by insulin. Pyruvate kinase is regulated by covalent modifications under the influence of the insulin / glucagon ratio.
Clinical Correlations[edit | edit source]
Relatively common enzymatic defects include congenital pyruvate kinase deficiency. Mainly erythrocytes are affected, in which less ATP is formed. This results in their lower ability to maintain the integrity of their membrane (active membrane transport) and their shape. The consequence is their increased breakdown - hemolytic anemia develops. In case of insufficient oxygenation of tissues (hypoxia), lactate is formed to an increased extent, which, as a relatively strong acid, causes acidification of the body - this drop in pH is called metabolic acidosis|lactic acidosis.