Fatty acids
Fatty acids (FA) are carboxylic acids with 4-26 carbons. They mostly have an even number of carbon atoms' (due to synthesis from two-carbon units - acetyl-CoA). They exist free (free fatty acids, VMK, free fatty acids, FFA), or are part of lipids (in the form of esters with alcohols - glycerol, sphingosine or cholesterolem).
Properties[edit | edit source]
They have amphipathic nature. It acts as surfactants, which are substances that reduce surface tension. Their solubility in water decreases with the length of the carbon chain (palmitic acid is more soluble than stearic acid), only butyric acid[1] is relatively soluble in water. Free fatty acids dissociate in an aqueous environment. They dissociate MK with a shorter carbon chain more easily. They are relatively well soluble in non-polar solvents.
Labeling of carbons and double bonds[edit | edit source]
Carbon numbering starts from the carbon bearing the carboxyl group – i.e. C1. The α carbon is located in close proximity to the carboxyl group - i.e. C2. ω carbon is the last carbon of a fatty acid - for example in palmitic acid, i.e. C16. ω3 means the third carbon from the end.
The position of the double bindings can be written in several ways:
1. Δ – the position of double bonds is given as a superscript.
- For example, Δ9, 12 indicates the position of the double bonds between carbons number 9 and 10, and 12 and 13 (calculated from the carboxyl group).
2. ω - indicates the position of the last double bond (farthest from the carboxyl group).
- For example, ω9 means a double bond on the 9th carbon from the end.
3. Simple enumeration - the position of the double bonds is given as the number of carbons (calculated from the carboxyl group) on which the double bonds are located. Often given after a semicolon (see abbreviated notation in the next paragraph).
Write[edit | edit source]
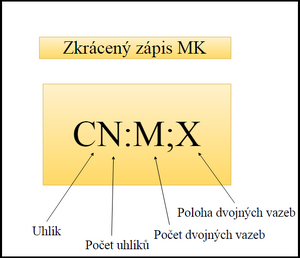
To describe fatty acids, ``abbreviated notations are used, which are composed of several numbers. The first number indicates the number of carbon atoms, the second (after the colon) the number of double bonds. The semicolon is followed by the double bond position. [2]
Arachidonic acid: C20:4;5,8,11,14
Splitting of fatty acids[edit | edit source]
- According to the presence of a double bond:
- saturated,
- unsaturated.
- By string length:
- short chain fatty acids (C4–C6);
- medium-chain fatty acids (C8–C10);
- long-chain fatty acids (C12–C18) → most common occurrence in higher animals;
- very long chain fatty acids (> C18).
- By string structure:
- linear - majority,
- branched – less common, e.g. isovaleric acid.
- According to whether the human body can synthesize them or must take them in food:
- essential,
- non-essential.
Saturated fatty acids[edit | edit source]
They contain no double bonds.
Table of saturated fatty acids[3]:
| Number of carbons | A trivial name | Systematic name |
|---|---|---|
| C4 | Buttery | Butane |
| C6 | Kapronová | Hexane |
| C8 | Caprylic | Octane |
| C10 | Kaprinová | Dean |
| C12 | Laura | Dodekanová |
| C14 | Myristová | Tetradecane |
| C16 | Palmita' | Hexadecane |
| C18 | Stear | Octadecane |
| C20 | Arachová | Eikosana |
| C22 | Behenová | Dokosanová |
| C24 | Lignocerous | Tetracosane |
| C26 | Cerotova | Hexacosane |
Unsaturated fatty acids[edit | edit source]
They contain one or more double bonds. Double bonds are not conjugated, but are isolated - separated by methylene groups (-CH2−). MKs with one double bond are referred to as monoenes (also monounsaturated). MK with two or more double bonds are referred to as polyene (also polyunsaturated) - e.g. diene, triene...
Table of unsaturated fatty acids[3]:
| Number of carbons and double bonds | Trivial name | Omega Series | Position of double bonds (all cis, with 1 exception) |
|---|---|---|---|
| C16:1 | Palm oil | ω7 | Δ9 |
| C18:1 | Oil' | ω9 | Δ9 |
| C18:1 | Elaidová | ω9 | Δ9 (trans) |
| C24:1 | Nervous | ω9 | Δ15 |
| C18:2 | Linoleic | ω6 | Δ9, 12 |
| C18:3 | α-linolenic | ω3 | Δ9, 12, 15 |
| C18:3 | γ-linolenic | ω6 | Δ6, 9, 12 |
| C20:4 | Arachidonic | ω6 | Δ5, 8, 11, 14 |
Cis/trans isomerism[edit | edit source]
It exists in unsaturated MKs due to the presence of a double bond around which the atoms cannot freely rotate. This isomerism depends on the orientation of the atoms around the axis passing through the double bond.
- Trans: each MK residue is on the opposite side of a double bond, eg elaidic acid.
- Cis: both MK residues are on the same side of the double bond, eg oleic acid.
Most unsaturated MKs have a double bond in the cis-configuration. The cis-configuration is important for the spatial arrangement of lipid molecules in cell membranes → MKs with double bonds in cis-configuration occupy more space and this makes membranes more fluid . MKs with double bonds in the trans configuration are found in some foods and are associated with an increased risk of cardiovascular disease and diabetes mellitus.
Essential (essential) fatty acids[edit | edit source]
It is necessary to supply them with food, because the human body is not able to create them. These include MKs with several double bonds (e.g. linoleic, linolenic and arachidonic acids). It is not possible to insert a double bond behind C9 in the human body, so we only synthesize ω9 unsaturated MK. ω3 and ω6 unsaturated MK must be taken in food. However, arachidonic acid is not a necessary part of food, as our body can synthesize it from other essential MKs (linoleic and linolenic acids).
Non-essential fatty acids[edit | edit source]
The human body can synthesize them and they are therefore not a necessary part of food. Examples are saturated MK and ω9 unsaturated MK' (i.e. palmitic acid, stearic acid, oleic acid...).
Importance of fatty acids[edit | edit source]
- They are part of many lipids.
- Energy source.
- Derivatives of polyene fatty acids with 20 carbon atoms – arachidonic acid, eicosapentaenoic acid and dihomo-γ-linolenic acid – are significant. These derivatives are called eicosanoids.
 For more information see Eicosanoids.
For more information see Eicosanoids.
Links[edit | edit source]
Related Articles[edit | edit source]
References[edit | edit source]
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedMatuš - ↑ KOOLMAN, Jan – RÖHM, Klaus-Heinrich. Color Atlas of Biochemistry. 1. edition. Prague : Grada, 2012. 512 pp. ISBN 978-80-247-2977-0.
- ↑ a b MATOUŠ, Bohuslav, et al. Basics of medical chemistry and biochemistry. 1. edition. Prague : Galen, 2010. 540 pp. ISBN 978-80-7262-702-8.
References[edit | edit source]
- ws:Mastné kyseliny
- MATOUŠ, Bohuslav, et al. Basics of medical chemistry and biochemistry. 2010. edition. Prague : Galen, 2010. ISBN 978-80-7262-702-8.
- MURRAY, Robert K. (Robert Kincaid). Harper’s illustrated biochemistry. 28. edition. New York : McGraw-Hill, Medical, 2009. ISBN 978-0-07-162591-3.
- KOOLMAN, Jan – RÖHM, Klaus-Heinrich. Color Atlas of Biochemistry. 1. edition. Prague : Grada, 2012. 512 pp. ISBN 978-80-247-2977-0.
- BAYNES, John W – DOMINICZAK, Marek H. Medical biochemistry. 3. edition. Philadelphia : Elsevier Mosby, 2009. ISBN 978-0-323-05371-6.