Anticoagulants
Anticoagulants are drugs that reduce blood clotting. By their action, they block coagulation factors, and thus coagulation itself (in layman's terms, "blood thinning" is referred to). They are mainly used for the prophylaxis of venous and intracardiac thrombosis and subsequent embolism. They are used in the laboratory as anticoagulants.
The ideal anticoagulant should:
- be applicable p.o. parenterally;
- have a rapid onset of action;
- have predictable properties and therefore fixed dosing;
- be safe;
- have an antidote available;
- be free from interactions with other drugs or foods.
Classification[edit | edit source]
Anticoagulants can be divided into substances used in vivo (patient) and substances used in vitro (laboratory).
| Anticoagulants used in vivo | Anticoagulants used in vitro |
|---|---|
|
|
Furthermore, anticoagulants can be divided into direct and indirect according to their mechanism of action. Direct anticoagulants inactivate the coagulation factors themselves present in plasma, while indirect anticoagulants affect coagulation factors by reducing their production in the liver.
| Direct anticoagulants | Indirect anticoagulants | |
|---|---|---|
| Direct inhibitors
thrombin / factor Xa |
Indirect inhibitors
thrombin / factor Xa | |
|
|
|
Direct anticoagulants[edit | edit source]
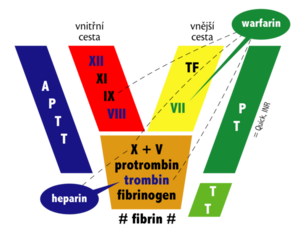
Direct anticoagulants block mainly thrombin and / or factor Xa. Thrombin is a key protein in the coagulation cascade that activates a number of coagulation factors and, in particular, catalyzes the conversion of fibrinogen to insoluble fibrin.
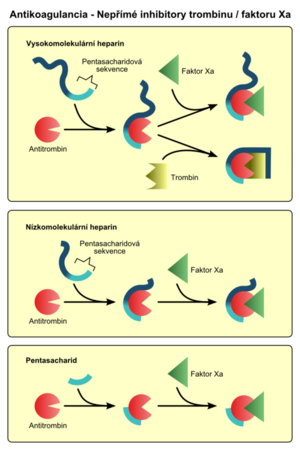
- Indirect thrombin / factor Xa inhibitors act by activating the natural thrombin inhibitor - antithrombin (AT III). They are therefore dependent on the presence of endogenous inhibitors. This is how heparins, for example, work.
- Direct thrombin / factor Xa inhibitors bind to thrombin or factor Xa and thereby block their function. These include xabans, gatrans and hirudins.
Heparin and its derivatives[edit | edit source]
Heparin is a mixture of acidic mucopolysaccharides commonly found in the body. Unfractionated heparin, low molecular weight and pentasaccharides are used therapeutically.
Unfractionated heparin[edit | edit source]
xxx
Unfractionated (natural) heparin activates AT III, which irreversibly inactivates thrombin and some other coagulation factors (eg factor Xa). It has the following features:
- does not cross the placenta and is therefore suitable for use in pregnancy;
- has a specific antidote - protamine,
but
- poorly absorbed, administered i.v. (in s.c. application, the effect begins after about 2 hours)[1];
- it has a short-term effect and has variable biodegradation, therefore APTT should be checked regularly after 6 hours;
- may induce thrombocytopenia (immune mechanism?).
Side effects: bleeding, allergic reaction, osteoporosis (long-term use).
Low molecular weight heparins[edit | edit source]
Low molecular weight heparins (LMWH) have shorter chains formed by the breakdown of heparin. They act similarly to unfractionated heparin (but inactivate mainly factor Xa), but they are safer, have a better anticoagulant effect, fewer side effects and more favorable pharmacokinetics. Low molecular weight heparins:
- are better absorbed, applied s.c. (mostly in the abdomen);
- have a longer effect;
- have a lower risk of induced thrombocytopenia;
- does not need to be checked by APTT,
but
- are sparingly neutralizable by protamine
- and have prescription restrictions in renal insufficiency (GFR <50 ml / min)
In practice, enoxaparin in particular is used.
Indications: prevention and treatment of thrombosis.
Contraindications: bleeding conditions.
Must not be given i.m. due to the risk of severe muscle bleeding.
A cheaper option with similar properties is bemiparin, but there are not enough studies.
Pentasaccharides[edit | edit source]
Pentasaccharides are synthetically prepared chains of five saccharide units, derived from heparin. They inactivate factor Xa through AT III. Pentasaccharides:
- they have a wider therapeutic window - they are safer and have a simple dosage;
- have a long and predictable effect,
but
- they are expensive (about 2 times more expensive against low molecular weight heparins);
- they do not have a specific antidote.
An example of a substance used is fondaparinux, idraparinux is under development.
Hirudins[edit | edit source]
Hirudin is a natural anticoagulant peptide produced by leeches (Hirudo medicinalis). It has good properties, but it is expensive and is not used in the Czech Republic. Several manufacturers currently supply recombinantly produced hirudin derivatives which, unlike the naturally occurring peptide, lack a sulphate group - desirudin.[2]
NOAC (novel oral anticoagulants) - new oral anticoagulants[edit | edit source]
Gatrans[edit | edit source]
Gatrans directly inhibit thrombin. Dabigatran is registered with us. It is administered p.o., has a rapid onset of action and a long-lasting effect. It can be used in the prophylaxis of thromboembolic disease (comparable to enoxaparin) and atrial fibrillation (better than warfarin). It is used in orthopedic indications and in the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation.
The promising dabigatran precursor ximelagatran was withdrawn from clinical practice in 2006 due to hepatotoxicity.[3]
Xabans directly inhibit factor Xa. Rivaroxaban has been registered with us since 2009. It is given p.o., it is predictable, the indications are similar to dabigatran. Newer is then registered apixaban.
Indirect anticoagulants[edit | edit source]
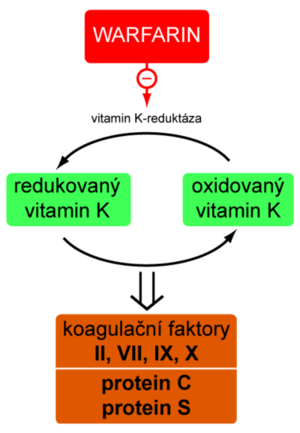
Indirect anticoagulants are competitive vitamin K antagonists (they have a similar structure). The result of their action is a reduction in the production of vitamin K-dependent coagulation factors.
Reduced vitamin K is oxidized during the synthesis of factors II, VII, IX, X and anticoagulant proteins C and S. The oxidized form must then regenerate vitamin K reductase - an enzyme that is the target structure blocked by indirect anticoagulants. From the above, it is clear that these substances are effective only in vivo. The peak of the anticoagulant effect starts in about 3 days.
The effectiveness of anticoagulant therapy may be affected by the amount of vitamin K in your diet or its absorption. In addition to food interactions, there are a number of drug interactions based on influencing drug biotransformation, synthesis and degradation of coagulation factors, or displacement of the drug from albumin binding.
Bleeding is an adverse effect of indirect anticoagulants. If it occurs, the drug should be discontinued; it is possible to administer vitamin K, plasma or a coagulation factor concentrate.
Warfarin[edit | edit source]
Template:See Warfarin for more information.
Warfarin is a coumarin derivative. For a long time, it was more or less the only oral anticoagulant available. It was initially used as a rat poison, but its potential use was revealed in a suicide attempt that was monitored by a blood clotting disorder.
- Variability
There are large interindividual differences in the pharmacokinetics of warfarin, therefore the dose should be strictly individualized. The vitamin K reductase and CYP2C9 polymorphisms apply. Drugs (especially amiodarone, fluvastatin, clopidogrel, non-steroidal anti-inflammatory drugs, a) and foods containing vitamin K (leafy vegetables, meat of cattle supplemented with vitamin K) interact. Therefore, the proportion of these foods in the diet should be as constant as possible.
CYP2C9 polymorphism can now be routinely determined in the laboratory.
- Treatment management
Warfarin has a short-term procoagulant effect at the beginning of treatment, as protein C and S synthesis are somewhat reduced. This period should therefore be covered by low molecular weight heparin. It starts at a dose of 5 mg / day (with LMWH overlap). The dosage is then adjusted according to the INR (Quick) results.
As already mentioned, the effect of warfarin fluctuates. Therefore, it is necessary to regularly check the INR, which should be in the range of 2-3.5. Measurements should be performed once every 3-5 days, in stable patients (ie twice in a row) once every 4 weeks. The current trend is home monitoring once a week.
Indications: prophylaxis of thrombosis and embolism in atrial fibrillation, after implantation of mechanical valve prostheses and in phlebothrombosis.
Contraindications: bleeding conditions, pregnancy (at doses above 5 mg / day conversion to LMWH).
When bleeding, it is necessary to interrupt the treatment, or to administer fresh frozen plasma, vitamin K or a complex of coagulation factors.
Ethyl biscum acetate (Pelentan®) is the original Czechoslovak preparation. It is very unstable and is no longer registered in the Czech Republic.[1]
Links[edit | edit source]
Related articles[edit | edit source]
- Prothromplex
- Heparin
- Warfarin
- Fibrinolytics
- Hemocoagulation
- Circulating anticoagulants
- Thrombosis
- Bleeding examination
- Blood clotting test
Source[edit | edit source]
- BULTAS, Jan. Kurz Farmakoterapie kardiovaskulárních chorob. 3. LF UK, 2010.
Reference[edit | edit source]
- ↑ a b LINCOVÁ, Dagmar a Hassan FARGHALI, et al. Základní a aplikovaná farmakologie. 2.. vydání. Praha : Galén, 2007. 672 s. s. 272–277. ISBN 978-80-7262-373-0.
- ↑ MAREK, Josef, et al. Farmakoterapie vnitřních nemocí. 3.. vydání. Praha : Grada Publishing, 2005. 773 s. s. 302-303. ISBN 80-247-0839-6.
- ↑ NOVOTNÝ, Jan. Nové antitrombotické léky. Interní medicína pro praxi [online]. 2006, roč. 8, no. 7–8, s. 327–329, dostupné také z <http://www.solen.cz/pdfs/int/2006/07/04.pdf>. ISSN 1212-7299.
References[edit | edit source]
- LINCOVÁ, Dagmar a Hassan FARGHALI, et al. Základní a aplikovaná farmakologie. 2.. vydání. Praha : Galén, 2007. 672 s. s. 272–277. ISBN 978-80-7262-373-0
- TROJAN, Stanislav, et al. Lékařská fyziologie. 4. vydání. Praha : Grada, 2004. 772 s. ISBN 80-247-0512-5.
- HYNIE, Sixtus. Farmakologie v kostce. 2. vydání. Praha : Triton, 2000. 520 s. ISBN 80-7254-181-1.