Amplification and expression of the isolated gene in the host cell
A major advance was the introduction of a method by which an isolated or synthetic gene could be multiplied. Some genes are only found in a single copy in a cell. Only by its laboratory multiplication is sufficient material available for the necessary analysis and therapeutic or biotechnological use.
Vector Synthesis[edit | edit source]
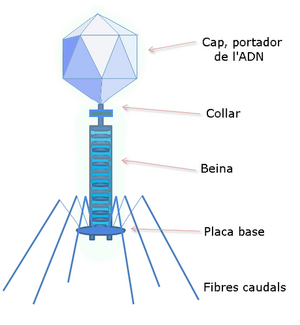
DNA , which is to be multiplied in the host cell, enters it quite exceptionally. It is necessary to incorporate it into a larger DNA molecule that has this property and which eventually integrates into the host genome . We call these carriers of artificial genes vectors . It is most often plasmid or viral DNA. Plasmid is a natural extrachromosomal element, circular dsDNA. Of the various types of these elements, those whose genes are carriers of the resistance of the host bacteria to some antibiotic are used in particular . A plasmid, for example, contains a gene for an enzyme breaking down a certain antibiotic. If a bacterium takes up such a plasmid, it becomes resistant to that antibiotic. One of the viruses (λ-phage or retrovirus ) is usually a larger vector with different properties. Its DNA is multiplied in the host cell, or integrated into the host genome.
Both the selected vector and the studied gene are provided with cohesive ends using restrictase or synthetically, with the help of which the gene is incorporated into the vector and covalently linked by DNA ligase. A vector carrying an exogenous gene is a recombined DNA with the original properties of the vector.
Selection of the host cell with the received new gene[edit | edit source]
Even under the most suitable conditions, exogenous (vector) DNA will be accepted by only a part of the used cells, and only this part needs to be recognized and used for the next procedure.
In the case of plasmid vectors, this problem is solved by the so-called insertional inactivation of the plasmid gene responsible for the antibiotic resistance of the host bacterium. Plasmid pBR322 contains an ampicillin (AMP) resistance gene and a tetracycline resistance gene(TC). The tetracycline resistance gene is cleaved with a suitable restrictase, for example, and the studied gene is inserted into this site. Acceptance of the plasmid into the bacterium is advantageously signaled by its resistance to antibiotics. Bacteria that have not taken up any plasmid are resistant to both antibiotics, bacteria containing a plasmid that failed to incorporate the new gene will be resistant to both antibiotics. Only bacteria carrying a plasmid with the new gene incorporated will show insertional inactivation, i.e. they will be resistant to ampicillin and sensitive to tetracycline. Only those are important to multiply and further use.
Gene cloning [ edit | edit source ][edit | edit source]
There are also other ways to determine the successful introduction of the gene into the host cell, e.g. using a complementary test, if the vector was a virus . The method is used in the reproduction and cloning of a gene , which is found even in a single copy in the studied huge genome. First, the gene must be found in the vast genome. The total genomic DNA is therefore cleaved by restrictases into a large number of fragments with cohesive ends, with the help of which the fragments are inserted into a vector, e.g. phage A. Each phage in the obtained preparation may contain a different fragment of the studied genome. The phages multiply in E. coli , creating a so-called genomic library, which can be preserved and any phage with a fragment of the studied genome can be multiplied at any time. It is important to find in this library a phage containing exactly the section of the studied genome that is needed. A diluted phage suspension is poured onto an agar covered with a layer of bacteria. In places of infection, circular clearings appear - plaques , created by the gradual lysis of bacteria attacked by phages. Plaques contain huge numbers of identical phage particles. This culture is printed on a nitrocellulose membrane, the bacteria and phages are lysed with NaOH, which simultaneously denatures their DNA. A plaque containing a complementary DNA sequence can then be found on this preparation using a suitable probe. By the way, in this way, the missing genes of the genome of a patient with a hereditary disorder can be diagnosed . In the original culture (matrix ) phages from the identified plaque can be transferred to fresh bacteria and multiplied with the gene that was introduced into them in the form of a restriction fragment. The gene was thus selected from the vast number of other genes and DNA sequences present and multiplied (cloned).
The required sample can be constructed artificially based on the knowledge of the amino acid sequence of the studied protein , or it can be obtained by reverse transcription of the relevant mRNA using a viral RNA-dependent DNA polymerase. The result is the so-called cDNA , which in eukaryotes can differ from the corresponding genomic DNA by the absence of introns.
Creating conditions for the expression of an artificial or rearranged gene in a host cell[edit | edit source]
The recombinant DNA molecule can be used to direct protein synthesis in the host cell. In this way, the genomic function of the patient's cells can be altered or a large amount of protein can be produced biotechnologically in vitro . However, the conditions for gene expression and product synthesis must be created. Then the mammalian protein can even be synthesized in a bacterium. Insulin mRNA was isolated from the pancreas, insulin cDNA was prepared by reverse transcription, incorporated into a plasmid and transferred to E. coli . The bacteria produced proinsulin. Several conditions had to be met in order for the foreign protein to form in the bacterium:
- The vector had to be constructed so that the gene was incorporated in the correct reading frame.
- It was advantageous to insert a powerful promoter in front of the gene, which rapidly initiated transcription .
- A sequence was inserted in front of the gene close to the initiation codon, which was the binding site of the mRNA to the ribosome.
The gene was integrated into the vector in the form of cDNA, so it was not interrupted by introns like the original gene in the pancreas. This circumvents the problem that the bacterium is unable to splice eukaryotic pre-mRNAs.
It should be noted that the bacteria synthesized proinsulin. It does not have enzymes for post-translational protein modification. Today, a significant part of the proinsulin needed for the therapy of diabetics is produced in the described manner. There are also methods for introducing exogenous DNA into a eukaryotic cell, where the aforementioned mechanisms for post-transcriptional and post-translational modification of products are not missing. In these systems, DNA is introduced into the cell using calcium phosphate, microinjection into the host cell, or using retroviruses as vectors. The rat gene for growth hormone was introduced into mouse germ cells. The mice grew to double their size (transgenic animals).
The first successes were also noted by botanists when they succeeded in introducing foreign genes into plant cells. This research is supposed to solve the genetic breeding of plants, ensure their independence from nitrogen fertilizers and basically ensure the nutrition of mankind.
According to some futurologists, thanks to advances in the biochemistry of nucleic acids, humanity is moving from a technical revolution to a biological revolution. Will it bring less worry and more benefits?
Links[edit | edit source]
Related Articles[edit | edit source]
- Biochemistry of genetic engineering
- DNA cleavage
- Separation of DNA fragments by electrophoresis
- Identification of restriction fragments
- Synthesis of artificial DNA
Source[edit | edit source]
- ŠTIPEK, Stanislav. Brief biochemistry: storage and expression of genetic information. 1st edition. Prague: Medprint, 1998. ISBN 80-902036-2-0 .
References[edit | edit source]
- ŠTIPEK, Stanislav. Brief biochemistry: storage and expression of genetic information. 1st edition. Prague: Medprint, 1998. ISBN 80-902036-2-0 .