Urea cycle disorders
__CONTENTLESS__
'Disorders of the urea cycle (small Krebs cycle, ornithine, ureosynthetic cycle) form a group of enzymatic disorders, the result of which is the accumulation of nitrogen in the form of ammonia, which is very toxic to the organism and causes irreversible brain damage.
The clinical manifestation of these diseases is usually already in the first days of life. Hyperammonemia causes convulsions, vomiting, and coma. In older children, these disorders are most often manifested by psychomotor retardation, failure to thrive, vomiting, behavioral disorders, repeated cerebellar ataxias and headaches.
In any patient with neurological symptoms of unknown origin, it is necessary to monitor the level of ammonia in the blood. The frequency of urea cycle disorders is approximately 1:30 000.[1] [2]
Pathogenesis[edit | edit source]
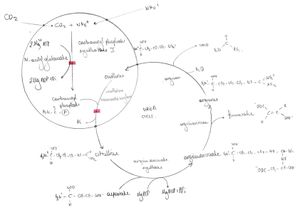
Urea cycle serves to excrete excess nitrogen (ammonia) in the form of urea. Urea is non-toxic, well soluble in water and diffusible. Urea is the main organic component of urine.
If the urea cycle is broken due to an enzymatic defect, hyperammonemia develops, accumulation of amino acids before the enzymatic block and, conversely, a decrease in the concentration of amino acids after the enzymatic block.
Plasma glutamine levels are also usually elevated. This is due to the fact that in this case an alternative way of converting ammonia is used, which is converted into glutamine with the help of glutamine synthetase and the substrate glutamate. The increased content of glutamine in astrocytes leads to their swelling and "brain edema" due to the osmotic effect.
When carbamoyl phosphate accumulates, orotic acid is formed, which is an important diagnostic marker. It is increased in disorders of all enzymes except CPS1, when even carbamoyl phosphate is not formed, therefore conversion is not possible.[2]
Division[edit | edit source]
It includes 5 hereditary disorders:
| damaged enzyme | location | inheritance type | incidence | OMIT | links | |
|---|---|---|---|---|---|---|
| Hyperammonemia I | carbamoyl phosphate synthetase (CPS1) | mitochondria | AR hereditary | rare (about 24 cases) | #237300 | [1] |
| Hyperammonemia II | ornithine carbamoyltransferase (OTC) | mitochondria | X-linked, manifestations can also be found in heterozygous girls | #311250 | [2] | |
| Citrullinemia | arginine succinate synthetase (ASS) | cytosol | AR hereditary | 1:70 000-1:100 000 | #215700 | [3] |
| Arginine succinaturia | arginine succinate lyase (ASL) | cytosol | AR hereditary | 1:70 000-1:100 000 | #207900 | [4] |
| Argininemia | arginase (ARG1) | cytosol | AR hereditary | rare (50 cases) | #207800 | [5] |
Clinical picture[edit | edit source]
 Urea cycle disorders usually occur in 2 forms – early and late.
Urea cycle disorders usually occur in 2 forms – early and late.
Early forms present shortly after birth with hyperammonemic coma, metabolic acidosis, hepatic failure, convulsions, and cerebral edema.
'Late forms are manifested by loss of appetite, vomiting, failure to thrive, hypotonia and disorders of psychomotor development.
Hyperammonemia (Type I)[edit | edit source]
It is a carbamoyl phosphate synthetase' defect that occurs in two forms: severe (lethal neonatal) and milder with later onset.
The lethal neonatal form' is manifested by severe brain damage, hyperammonemic coma and ketoacidosis. In the milder form hyperammonemic coma, Reye-like syndrome, vomiting, hypotonia, failure to thrive and psychomotor retardation may occur[3].
When analyzing the laboratory examination, we find low concentrations of arginine and citrulline''''''''''''''''''high concentrations of glutamine'. Conversely, uracil and orotic acid are normal.
Citrullinemia (Type I)[edit | edit source]
It is a defect of argininosuccinate synthase occurring in two forms. The first is neonatal, manifested by hyperammonemic coma and lactic acidosis. The second form is ``chronic juvenile, whose symptoms are loss of appetite, vomiting, hypotonia, growth and psychomotor retardation, and convulsions.
We distinguish two more types of citrullinemia. Type II' is characterized by a deficiency of the mitochondrial transporter of aspartate and glutamate (citrine), resulting in an intramitochondrial aspartate deficiency. Type III is characterized by a partial deficiency of arginine succinate synthetase with high residual enzyme activity [4]
In the laboratory, we find low concentrations of arginine, but high concentrations of citrulline and glutamine, uracil and orotic acid are elevated.
Arginine succinaturia[edit | edit source]
It is an arginine succinate lyase defect that occurs in two forms, early and late. The early form' presents with severe hyperammonemic coma shortly after birth and is often fatal. In the late form we can observe hypotonia, failure to thrive, loss of appetite, chronic vomiting and behavioral disorders during childhood. Other manifestations may be hepatomegaly and brittleness of hair (trichorrhexis nodosa)<ref>https://www.orpha.net/consor/cVgi-bin/OC_Exp.php?lng=EN&Expert=23</ ref>.
In the laboratory, we find a "low concentration of arginine" and an "increased concentration of glutamine and citrulline", in the urine we demonstrate an "increase in the concentration of orotic acid and uracil".
Argininemia[edit | edit source]
It is an arginase I' defect whose symptoms include spastic diplegia, epilepsy, psychomotor retardation, hyperactivity, irritability, inconsolable crying, anorexia, vomiting, and rarely symptomatic hyperammonemia progressing into a coma.
In the laboratory, we will demonstrate "hyperargininemia" and "increased excretion of orotic acid" in the urine. The leading symptom is "hyperammonemia".
If we look at the ABR, we first find respiratory alkalosis and later metabolic acidosis.
Another important indicator is "amino acids in plasma" (chromatography), where in the results we find an increased concentration of glutamine and glutamic acid and little arginine (except for argininemia), as well as an increased concentration of amino acids before the enzymatic defect and a reduced concentration of amino acids behind the defect ( e.g. low citrulline and high orotate → OTC - same as any enzyme block).
Urinary orotic acid is increased in disorders of all enzymes except CPS1.
We perform determination of enzymatic activity from liver tissue and analysis of mutations[2].
Differential diagnosis of hyperammonemia[edit | edit source]
Disorders can be divided into congenital and acquired.
Congenital defects include urea cycle disorders, organic aciduria, disorders of fatty acid transport or oxidation, hyperinsulinism, and hyperammonemic syndrome.
Acquired include Rey's syndrome, liver failure of other etiologies, transient hyperammonemia of the newborn (mainly in NNPH). Treatment is carried out with the help of valproate em[2].
Therapy[edit | edit source]
First aid consists in converting catabolism into anabolism (i.v. high doses of glucose with insulin, high-calorie parenteral nutrition) and detoxification. Sodium benzoate'' activates alternative nitrogen excretion pathways. Phenylbutyrate, which is metabolized to phenylacetate, binds glutamine and allows it to be excreted by the kidneys. In case of impaired consciousness, an elimination method (hemodialysis, hemodiafiltration) should be used to reduce ammonia. Next, we substitute amino acids (usually arginine and citrulline - valid only in selected defects).
Protein intake must be "reduced" to 0-1.2 g/kg/day throughout life, and at the same time their substitution must occur with the help of mixtures of essential amino acids. In case of severe metabolic impairment, liver transplantation[2] is necessary.
Prognosis[edit | edit source]
With early therapy (except severe forms of OTC) it can be good, with the development of severe hyperammonic coma (mostly above 300 μmol/l ammonia with a norm of around 50 to 70 μmol/l) in the newborn age there is a high risk of disability[2]. Template:Do not print
- ↑ TASKER, Robert C. – MCCLURE, Robert J. – ACERINI, Carlo L.. Oxford Handbook of Paediatrics. 1. edition. New York : Oxford University Press, 2008. 936 pp. ISBN 978-0-19-856573-4.
- ↑ a b c d e f MUNTAU, Ania Carolina. Pediatrics. 4. edition. Prague : Grada, 2009. pp. 111-112. ISBN 978-80-247-2525-3.
- ↑ https://www.orpha.net/consor/cgi-bin/OC_Exp.php? lng=EN&Expert=147
- ↑ https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=187