Polysaccharides
They are organic compounds composed of 10 or more monosaccharides, which are connected by a glycosidic bond. They have a storage and construction function.
Ballast substances (vegetable fibres, dietary fibres) - these are carbohydrates that are not enzymatically digested in the small intestine and therefore reach the large intestine - the most important: cellulose, hemicellulose, pectin, resistant starch (this also includes substances with non-fibrous structure, so the other names should not be used).
Classification[edit | edit source]
According to bound monosaccharides:
homopolysaccharides (homoglycans) – consist only of molecules of a single monosaccharide.
- glucans: α-glucans (amylose) a β-glucans - celulosa
- fructans
heteropolysaccharides (heteroglycans) – contain several types of monomeric units or their derivatives in the molecule. This includes most polysaccharides, for example arabinoxylans.
By origin:
- natural − glycans of plants, animals;
- additive − glycans of algae, fungi, microbes, modified plant glycans.
According to basic functions:
- storage (reserve) – glycogen, starch, non-starch glycans;
- building (structural) – chitin, cellulose and associated glycans;
- with other functions - gum arabic, okra (water management, tissue protection).
According to to application in nutrition:
- usable − starch, glycogen (animal starch);
- unusable − dietary fiber (the human body cannot use it because it lacks the enzymes needed for digestion); these polysaccharides primarily include cellulose, hemicellulose and pectins.
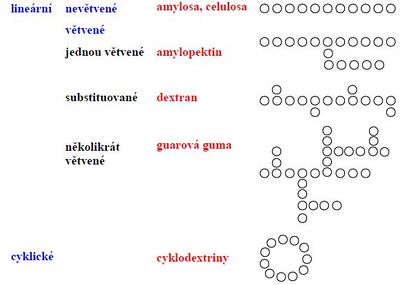
According to to the type of chain:
Starch[edit | edit source]
The most common storage polysaccharide of plants. It is made up of α-glucoside hains. It is found in cereals, potatoes, legumes and other vegetables. Starch grains (granules) are formations in which starch is stored. Inside the cells, it is concentrated in the so-called amyloplates (in roots, tubers, seeds and fruits) and in chloroplasts (in tissues where photosynthesis takes place). The main components are amylose and amylopectin.
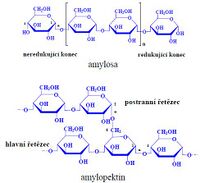
- Amylose (15-20 %)
It is a linear polymer that consists of alpha-D-glucose (several hundred units connected by α(1→4) bond). It belongs to glucans. It is soluble in water (especially in hot water), the solutions are very viscous (less viscous than amylopectin). The macromolecule forms a left-handed helix. Number of Glu units 1000 – 4500. The amylose of cereal starches contains 1000 to 2000 glucose units in the chain, in potato starch up to 4500 glucose units.
- Amylopectin (80-85 %)
It consists of branched chains composed of 24-30 glucose units (a total of 50,000 to 1,000,000 glucose units in one molecule) and the chain is highly branched (primarily α(1→4)-glycosidic bonds, with its abundant branching (α(1→6) bonds ) is structurally similar to the animal storage polysaccharide glycogen). It is insoluble in cold water , but lubricates when heated and forms a viscous solution that hardens into a gel after cooling.
Properties of starch
Starch has the ability to bind water (starch swelling).
E.g. at 70°C, it can absorb up to 25 times its weight. With a sufficient amount of water, starch greases are formed, which, after cooling, form gels, if there is not enough water, doughs are formed. Starch grains take up water from the air at a rate of about 0.2 g of water per 1 g of dry starch without changing their volume or physical properties, a process known as imbibition. From a chemical point of view, there are about 1.5 water molecules per 1 glucose unit. 1 g of initially dry starch can reach a final weight of up to 25 g and occupy a volume of up to 200 ml. The resulting viscosity of starch oil depends on the ratio of amylose and amylopectin (the higher the proportion of amylopectin, the more viscous the starch solution). The gelatinization temperature is not an exact value, it is usually stated for the given type of starch in the range of about 10 - 15°C (gelatinization usually occurs in the temperature range of 50 - 80°C). By heating starch granules in water, amylose molecules are gradually released into the water as the temperature increases, in this way amylose can be separated from amylopectin. Amylose is already released at swelling temperatures, so the gelatinization process does not necessarily have to occur. The gelatinization temperature of corn starch is in the range from 62 to 70°C, rice from 68 to 78°C, wheat from 52 to 64°C, rye from 57 to 70°C and potato from 52 to 68°C.
Starch digestion
The starch molecule is too large to pass through the intestinal wall into the blood. Due to the action of glycosidase enzymes (in saliva and in secretions of the pancreas and intestinal mucosa), short fragments are gradually split off from the surface of starch grains, and thus short chains (around ten glucose units) are formed. Gycosidases are highly selective enzymes, they only hydrolyze the α-glycosidic bonds of starch, while they cannot cleave the β-glycosidic bonds contained in cellulose (a person can digest grain, potatoes or rice, but not grass or leaves). These short glucose fragments are transported by blood to liver and muscle cells , where they are either directly incorporated into glycogen, or they are broken down into glucose , which the body further transforms for the simultaneous gain of a large amount of energy.
- Physiological malabsorption of starch - about 10% of the starch ingested in a mixed diet is resistant to α-amylase and reaches the large intestine, where it is destroyed by bacteria as a ballast substance.
- Modified starches - starch (e.g. potato, wheat, corn, rice) that has been modified using physico-chemical or enzymatic processes to achieve the desired properties. It is most often used as a stabilizer or thickener.
Glycogen[edit | edit source]
Storage polysaccharide of animal tissues (muscle, liver) - so-called animal starch. It has a more branched structure than amylopectin. One glycogen molecule consists of up to 120,000 glucose molecules - a chain of 10 – 18 α-D-glucopyranose residues (in an α(1→4) glucosidic bond, α(1→6) branching). It is stored in the form of granules in the cytoplasm of some cells of higher animals, especially in liver cells (human liver cells contain 18-20% of glycogen in dry matter) and muscles (muscle cells about 0.5-1%), but also in fungi and yeasts. Approximately 250-400 g of glycogen in reserve (1/3 in the liver, 2/3 in the muscles), athletes up to 800 g. The size of the glycogen reserves is influenced by diet (mainly diet containing carbohydrates). The supply is exhausted after 30-90 minutes of exercise, depending on the intensity.
- liver glycogen - maintains a stable blood sugar level (especially when fasting)
- muscle glycogen - immediately usable for muscle work as an immediate source of energy
Fiber[edit | edit source]
The polysaccharide components of fiber include cellulose, hemicelluloses, pectin substances, vegetable gums and mucilages, resistant starch, inulin, glycosaminoglycans of animal tissues and fungi.
Hemicelluloses include:
- xyloglucans (in vegetables and legumes)
- arabinoxylans and β-glucans (in cereals)
- galactomannans (in legumes)
Soluble fiber
It has the ability to absorb water, swell. It ferments in the digestive tract - it can be a source of energy. It regulates the digestion of fats and carbohydrates, binds water to itself and thereby gains volume - leading to a feeling of satiety. For the most part, it is a nutrient for the microbial flora in the digestive tract, it acts as a so-called prebiotic. Sources: legumes (peas, soybeans, beans), pods and flax seeds (source of soluble and insoluble fiber), oats, rye, barley, some fruits (apples and bananas) and berries, some vegetables (broccoli and carrots), root vegetables , potatoes (their skin contains insoluble fiber), psyllium seeds (only about ⅔ soluble fiber).
Insoluble fiber
It does not ferment in the digestive tract - it is not a source of energy. Increases the volume of contents in the intestines - shortens the time that food remains there. It is especially beneficial in the large intestine - thanks to the increase in the volume of the stool, it dilutes the waste substances that were created during digestion -> they then leave the digestive tract more easily, which is thus exposed to potentially dangerous substances for a shorter period of time. Sources: whole grain foods containing mainly husks, capsules and flax seeds (a source of soluble and insoluble fiber), cereal husks, bran, nuts and seeds, vegetables (green beans, cauliflower, zucchini, celery), peels of some fruits and tomatoes.
Inulin[edit | edit source]
It is a storage polysaccharide of some plants - it replaces starch as a storage substance. A chain of fructofuranose units (β(1→2) bond) terminated by glucose. The number of sugar units is around 30. There is great variability in individual species. There are up to 200 units. It is a crystalline substance soluble in water when hot.
Occurrence: chicory root, Jerusalem artichoke tubers, artichokes, asparagus, dahlia roots, dandelions. Unusable polysaccharide - not broken down by digestive enzymes (hydrolyzed only by plant inulins). It has prebiotic effects. The possibility of use for the production of fructose syrups.
Celullose[edit | edit source]
The main structural component of plant tissue (main polysaccharide of plant cell walls, algae). It is insoluble in water, in dilute solutions of acids and bases. It consists of 3,000 glucose molecules. It has a high ability to bind water - 1 g of cellulose binds 0.4 g of water. Humans (and many mammals) cannot digest cellulose - they lack hydrolase. It is a significant source of bulky indigestible residue in food.
Occurrence: fruits and vegetables 1-2% - legumes and cereals. It is the main component of pulp from which paper is made.
Hemicellulose[edit | edit source]
It occurs together with cellulose in plant cell walls. It consists of 150-200 glucose molecules. It binds water and cations (the ability to bind cations is affected by uronic acid). The following sugars are most often found in it - xylose, arabinose, mannose, uronic acid.
Pectins[edit | edit source]
D-galactopyranuronic acid units linked by an α(1→4) bond. Number of units in chain 25-100. It participates in the construction of plant tissues. Insoluble in water (so-called protopectins), they are soluble by the action of acids or protopectinases. They are able to bind water to each other - they participate in the management of cells with water. They are important during fruit ripening – the softening of the fruit is caused by the conversion of insoluble pectins into soluble ones. Sources: fruits and vegetables (main sources for insulation - apple pomace, citrus peels), legumes, oil seeds, sugar beet. They form gels and are used as gelling agents.
Consequences of changes in pectin substances
Insoluble pectin substances - the cause of the hardness and firmness of unripe fruits and vegetables. Softening occurs due to insufficient depolymerization during ripening. The post-harvest softening of the fruits means a deterioration of the shelf life. There are some measures to slow softening such as: thermal inactivation of pectolytic enzymes, addition of bivalent cation salts.
Glycosaminoglycans[edit | edit source]
Polysaccharides containing units of aminodeoxysugars or their acetyl derivatives. Occurrence in animal tissues and fungi. The group includes:
- chitin and chitosan
- so-called mucopolysaccharides - in addition to the amino sugar, they also contain another sugar unit (hexose or alduronic acid) and often a sulfate group, mucopolysaccharides. They form complex compounds with peptides and proteins (proteoglycans, mucoproteins), occur in epithelial and connective tissues (eg: chondroitin sulfates, keratan sulfate or hyaluronic acid), eye tissues (hyaluronic acid), liver, lungs (heparin).
Chitin
Structural polysaccharide of invertebrates - the main component of the cuticle of arthropods. Part of the cell wall of fungi. Use in surgical instruments. It occurs in crustaceans, molluscs, insects, fungi, yeasts and bacteria. The natural source for production is the shells of sea mussels. It is insoluble and indigestible. It is partially cleaved by the action of lysozyme.
Chitosan
Chemically modified chitin with a lower proportion of acetyl groups (5-25%). Soluble in water and acid solutions (the solutions are viscous), insoluble in alkaline environments. It coagulates in the presence of proteins, alginate. Use: emulsifier and dispersion stabilizer. It is indigestible. It lowers the level of fats and cholesterol in the blood serum.
Hyaluronic acid
Chains of alternating units of D-glucopyranuronic acid and N-acetyl-D-glucosamine linked by a β(1→3) bond. It is found in the skin, cartilage, synovial fluid and the vitreous of the eye. It is soluble in water. It forms very viscous solutions (significantly increases in volume after its dissolution). It is broken down by the effect of hyaluronidase (produced by bacteria, contained in snake venoms and insect venoms.
Chondroitin sulfates
It occurs in the skin, cartilage and in the glycoproteins of saliva.
Dermatan sulfate
It is found in the skin, tendons and blood vessels.
Keratin sulfate
It is found in the cartilage and cornea.
Heparin
The chains connect D-glucuron-2-sulfate and N-sulfo-D-glucosamine 6-sulfate by an α(1→4) bond, the position of the sulfate groups may differ in different types of heparin. It occurs in the liver and lungs. Prevents blood clotting (hemostasis), inhibits the conversion of prothrombin to thrombin.
Other representatives of polysaccharides[edit | edit source]
Vegetable Gums and Mucilages[edit | edit source]
Plant gums are solid rubbery substances created by the drying of plant juices (spits) flowing from plant tissues. Plant mucilages are slimy masses of various parts of some plants. Examples are gum arabic, larch gum, tragacanth, gum india, gum ghati, okra mucilage, baobab mucilage.
Seaweed polysaccharides[edit | edit source]
We divide into:
- agars, carrageenans, furcellaran – from red algae (Rhodophyceae)
- algins – from brown algae (Phaeophyceae).
We use them in the food industry as gelling agents, thickeners, stabilizers and emulsifiers.
Bacterial polysaccharides[edit | edit source]
Extracellular – they form a slimy envelope of cells:
- xanthan (xanthan gum) - use: thickener
- gellan (gellan gum) – cold gel formation
- dextran – emulsion stabilizer.
Intracellular polysaccharides have a structural or storage function.
Yeast and fungal polysaccharides[edit | edit source]
Representatives are Elsinan, pullulan, scleroglucan, β-glucans from various yeasts and higher fungi.
Links[edit | edit source]
Related Articles[edit | edit source]
References[edit | edit source]
- MURRAY, Robert K. – GRANNER, Daryl K. – MAYES, Peter A.. Harperova biochemie. 4. edition. Nakladatelství H+H, 2002. 872 pp. pp. 749. ISBN 80-7319-013-3.
- DAVÍDEK, Jiří. 6. POLYSACHARIDY [online]. [cit. 2012-03-12]. <https://el.lf1.cuni.cz/p55455514/>.
- VELÍŠEK, Jan – HAJŠLOVÁ, Jana. Chemie potravin. 2. 3. edition. OSSIS, 2009. ISBN 978-80-86659-17-6.
- MCMURRY, John. Organická chemie. 2. edition. Vutium, 2007. 572 pp. ISBN 978-80-214-4769-1.
- KASPER, Heinrich. Výživa v medicíně a dietetika. - edition. Grada, 2015. 572 pp. ISBN 9788024745336.
- INSTITUT GALENUS,. Škroby [online]. [cit. 2018-02-14]. <http://www.galenus.cz/clanky/vyziva/polysacharidy-skroby>.
- KOPLÍK, Richard. Polysacharidy: Studijní materiály k předmětu Chemie potravin [online]. [cit. 2018-02-15]. <https://web.vscht.cz/~koplikr/CHP_sacharidy_2.pdf>.
- DOLEŽAL, Marek. Polysacharidy: Přednášky předmětu Chemie potravin - sylaby [online]. [cit. 2018-02-15]. <https://web.vscht.cz/~dolezala/CHPS/06%20Polysacharidy.pdf>.