Carbohydrates
Carbohydrates (also sugars, Latin saccharum, Greek sákcharon) are basic component of all living organism and at the same time the largest group of organic substances, making up the largest proportion of organic matter on Earth. Animal tissues and their cells contain less carbohydrates than proteins and lipids (e.g. the human body contains about 2% carbohydrates in dry matter), plants contain 85 to 90% carbohydrates in dry matter.
Knowing the structure and properties of physiologically important carbohydrates is necessary to understand their role in the human body, where sugars are the most important energy source for cells. The daily intake of carbohydrates in humans is 300-500g, the body obtains them mainly in the form of polysaccharides (60% is starch), disaccharides (30% is (sucrose), the rest is made up of other disaccharides and monosaccharides.
Biomedical significance[edit | edit source]
- Carbohydrates are an important and fastest source of energy.
- Carbohydrates are metabolic intermediates for synthetic processes.
- They are part of nucleotides, RNA a DNA.
- They form the structural elements of the membranes of lower organisms and in the form of complex liposaccharides and glykoproteins or proteoglycans are part of membranes and tissues of animals and humans.
- They play an important role in internal and intercellular communication and immunity.
- Humans can synthesize carbohydrates (with the exception of vitamin C) mainly from amino acids. And therfore a low-carbohydrate and high-carbohydrate diet leads to nitrogen loss or metabolic acidosis.
Composition and distribution of carbohydrates[edit | edit source]
From a chemical point of view, carbohydrates are polyhydroxyaldehydes , polyhydroxyketones and polyhydroxyalcohols, which are divided into monosaccharides, oligosaccharides and polysaccharides.
- Monosaccharides – represent the simplest sugars, which are aldehyde or ketone derivatives of polyhydroxyalcohols with an unbranched chain. They contain at least 3 carbon atoms and at most 9 carbon atoms. These substances cannot be hydrolyzed into simpler carbohydrates.
- Oligosaccharides contain 2–10 monosaccharide units in the molecule covalently bound by an O-glycosidic bond. They are an important part of complex lipids and proteins, where as glycolipids or glycoproteins, perform a structural and regulatory function.
- Polysaccharides (glykans) - are formed by a large number of covalently bound monosaccharide units, reaching a molecular weight of up to several million daltons (Da). They are also a basic component of the cell walls of plants and bacteria (e.g. cellulose, chitin), where they fulfill a supporting function. Starch in plants and glycogen in animals serve as storage substances.
Carbohydrate structure[edit | edit source]
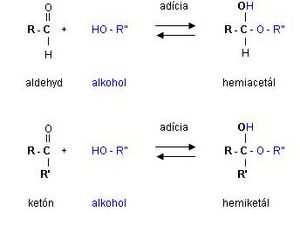
Fischer's formulas describe the typical reactions of the carbonyl group of monosaccharides, but they cannot explain the formation of so-called hemiacetals or hemiketals and the associated formation of a glycosidic bond. The carbonyl group is very reactive, so with a sufficient length of the carbon chain (pentose, hexose), the hydroxyl group of the given molecule can be added to it, i.e. intramolecularly, creating a cyclic hemiacetal or hemiketal form. The configuration of substituents on each carbon of carbohydrates in the cyclic form is conveniently shown by Haworth conformational formulas .
Carbohydrates with a six-membered ring that are derived from pyran are referred to as pyranoses , (the simplest compounds containing such a ring) by adding the suffix -ose . Similar saccharides with a five-membered ring are referred to as furanoses after the furan. Thus, the cyclic forms of glucose and fructose are glucopyranose and fructofuranose - the furanose rings are smaller than the pyranose rings.
The pyranose ring is six-membered, and therefore can have two basic conformations in space, stool and tub.
In nature, molar conformation prevails. The conformational structures show that the β-isomer is more stable because it has a bulkier –OH group in the equatorial position. Therefore, both anomers are not equally stable in solutions, and the α-anomer can change to the β-anomer. Cyclization of monosaccharides creates a new center of asymmetry at carbon C1 . The resulting two diastereoisomers are referred to as anomers and the hemiacetal or hemiketal carbon as anomeric .
In the anomer, the –OH group on the anomeric carbon is in the opposite position (below the plane) relative to the saccharide ring than the CH 2 OH group of the chiral center, determining the D or L- configuration (for hexoses on C 5 ). The second anomer is referred to as the β-form. For each ring form, there is a possibility of the formation of two α- and β- anomers, which are in equilibrium with each other. Each of the two anomers of D-glucose, like each pair of diastereomers, has different physical and chemical properties. The interconversion of tautomeric forms in solution to establish a dynamic equilibrium between them is referred to as mutarotation .
Carbohydrate reactions[edit | edit source]
Carbohydrate oxidation[edit | edit source]
Carbohydrates whose anomeric carbon atom is not part of a glycosidic bond are called reducing carbohydrates , because they retain the ability to oxidize to the corresponding acid and reduce mild oxidizing agents. The most common test (evidence) for the presence of reducing carbohydrates is the Fehling test (reduction of Cu 2+ with Fehling's solution). The presence of an aldehyde group in aldoses and the primary hydroxyl groups of aldoses and ketoses enables their further oxidation. By chemical or enzymatic oxidation of aldoses, the aldehyde group changes to a carboxyl one and so-called aldonic acids are formed (e.g. gluconic acid is formed by the oxidation of glucose ). The name aldonic acids of carbohydrates is a combination of the word acid and endings -onic to the root of the name of the corresponding saccharide.
Specific oxidation of the primary alcohol group of aldoses produces uronic acids; the names of which consist of the word acid and the -uronic ending of the name of the corresponding saccharide. An important component of polysaccharides are D-glucuronic, D-galacturonic and D-mannuronic acids.
Glucuronic acid is used in the detoxification of many harmful substances in the liver (it binds phenols, alcohols , benzoic acid and other toxic substances, creating less toxic glucuronides) and as a component of glycoproteins (mucoitinsulfuric acid, chondroitinsulfuric acid). Galacturonic acid is a component of pectin.
Both aldonic and uronic acids have a strong propensity for intramolecular esterification, which leads to cyclization and the formation of the respective lactones.
Ascorbic acid ( vitamin C ) is an α-lactone, synthesized by plants and most animals (except primates and guinea pigs). Reduction of glucuronic acid produces gulonic acid, which provides gulonolactone, an intermediate in the biosynthesis of ascorbic acid. Aldaric acids (e.g. glucaric acid) are formed by simultaneous oxidation of the aldehyde group and the primary alcohol group.
Oxidation reactios of glucose
By oxidizing the aldehyde group to a carboxyl group, the newly formed compound loses its ability to form cyclic (semi-acetal) forms. Of the mentioned oxidation products of monosaccharides, the ability to form hemiacetals is therefore preserved only in the case of uronic acids.
Ketoses under the action of strong oxidizing agents give hydroxydicarboxylic acids with a smaller number of carbons, because the carbon chain between the first carbon and the carbon of the keto group is split. L-ascorbic acid (L-gulonic ketocarboxylic acid dehydrolactone, vitamin C) can be oxidized to biologically inactive dehydroascorbic acid.
Carbohydrate reduction[edit | edit source]
Aldoses and ketoses can also be enzymatically reduced to polyhydroxyalcohols, the so-called alcoholic sugars - alditols , by the action of mild reducing conditions . Their names are formed by adding the ending -tol to the root of the name of the corresponding aldose. During the oxidation of ketoses, the carbon of the oxo group becomes asymmetric, which is why a mixture of two alcoholic sugars differing in the position of the hydrogen and the –OH group on this carbon is formed (D-glucitol and D-mannitol are formed from D-fructose).
Carbohydrate derivates[edit | edit source]
Formation of glykosides
A special group of monosaccharide ethers consists of ethers that are formed by esterification to hemiacetal hydroxyl group (i.e. on the C1 carbon of aldoses and the C2 carbon of ketoses), forming so-called glycosides, and their bond is called a glycosidic bond.
Many carbohydrates do not occur free in nature, but the hemiacetal hydroxyl group can be replaced by an organic component (e.g. alcohol, phenol, sterols, terpenic alcohols, hydroxy derivatives of heterocycles) and heteroglycosides are formed. As the hemiacetal hydroxyl group of a saccharide condenses with another monosaccharide, a homoglycoside (glycan) of the α- and β-glycoside type is formed.
The type of glycosidic bond is very important because enzymes distinguish it very strictly. Glycosides (glykys - Greek - sweet) do not have a free hemiacetal hydroxyl and therefore do not have reducing effects. According to the type of connecting atom, they are divided into O-glycosides, S-glycosides (the non-sugar residue is bound to the sugar molecule via the S atom) and N-glycosides (this includes nucleosides - part of nucleic acids, ATP). The glycosidic bond connecting the monosaccharide units of polysaccharides is actually similar to the peptide bond of proteins. Hydrolysis of glycosidic bonds is catalyzed by glycosidase enzymes, or starch and glycogen by α-amylase.
Formation of esters
Hydroxyl groups in monosaccharide molecules are also esterified (e.g. by organic acids, H3PO4 , CH3COOH). Carbohydrate esters with sulfuric acid are part of polysaccharides, especially glycosaminoglycans.
The most biologically significant are esters of monosaccharides with phosphoric acid. They represent activated forms of carbohydrates and are important intermediates of metabolic pathways, e.g. glyceraldehyde-3-phosphate and dihydroxyacetone phosphate occur in the metabolism of carbohydrates in every cell. Other important esters include, for example, glucose-1-phosphate (Glc-1-P) – Cori's ester, glucose-6-phosphate (Glc-6-P) – Robinson's ester, fructose-6-phosphate (Fru-6-P ) – Neuberg ester and, last but not least, fructose-1,6-bisphosphate (Fru-1,6-PP) – Harden-Young ester.
Deoxysaccharides
Reduction (deoxygenation) of the hydroxyl group of a monosaccharide produces a deoxysaccharide.
The most biologically significant representative of this group is D-2-deoxyribose, the sugar component of DNA.
6-deoxysaccharides (also called methylpentoses), L-rhamnose (6-deoxy-L-mannose) and L-fucose (6-deoxy-L-galactose) are also found in living nature, which are important components of bacterial cell walls and some polysaccharides, especially proteoglycans.
Aminosaccharides
By replacing one or more hydroxyl groups with an amino group (–NH 2 ), which is often acetylated, amino sugars or aminosaccharides. D-glucosamine (e.g. in chitin, some antibiotics) and D-galactosamine (e.g. in chondroitin sulfate of some cartilages and tendons) are components of many biologically important polysaccharides.
Oligosaccharides[edit | edit source]
Apart from glucose, the most common disaccharides and trisaccharides are found in nature. They are colorless, crystalline and sweet substances that are well soluble in water. Oligosaccharides occur in nature primarily as components of glycolipids and glycoproteins. Disaccharides are formed from two molecules of monosaccharides linked by an α- or β-glycosidic bond. It is cleaved by acid hydrolysis or enzymatically to release the corresponding monosaccharides. Non-reducing saccharides (sucrose and trehalose) are formed by joining monosaccharide units via hemiketal hydroxyls, and thus the possibility of oxidation of the carbonyl group is lost. The so-called reducing disaccharides (maltose, cellobiose, lactose) are formed by combining the hemiacetal hydroxyl of one monosaccharide molecule with an alcohol hydroxyl of the other monosaccharide.
Only 3 disaccharides occur freely in nature: sucrose, lactose and trehalose. Others are formed during the hydrolysis of polysaccharides and heteroglycosides.
The most widespread disaccharide is sucrose - O-α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside (beet or cane sugar), composed of α-D-glucopyranose and β-D-fructofuranose, with an O-glycosidic bond (1 →2) connects C1 on the glucose residue with C2 on the fructose residue. By binding, it loses its reducing power, which is indicated by the ending -id in the systematic name. Sucrose is found in all plant fruits and plant juices. It is used to sweeten food and drinks and as an ingredient in various liqueurs. Hydrolysis (in an acidic environment) of sucrose into glucose and fructose is accompanied by a change in optical rotation from dextrorotatory to levorotatory, as a result of the influence of strong levorotatory D-fructose. Consequently, sucrose hydrolyzate is sometimes referred to as invert sugar. Hydrolysis of sucrose can be carried out not only with the help of acids, but also enzymatically (with sucrose).
Polysaccharides[edit | edit source]
Polysaccharides, referred to as glycans, are composed of monosaccharides (more than 10) linked by glycosidic bonds. According to their structure, we divide them into homopolysaccharides (e.g. starch, glycogen, cellulose, fructan, inulin) and heteropolysaccharides (e.g. hemicelluloses, mucilages) based on whether they are composed of one or more types of monosaccharides. Although the sequence of monosaccharides in heteropolysaccharides can be variable, usually polysaccharides are composed of only a few types of monosaccharides that are linked in a repeating sequence. Thus, polysaccharides form both linear and branched polymers, since the glycosidic bond can originate from any hydroxyl group.
According to the biological function, we distinguish structural (e.g. cellulose, chitin) and storage polysaccharides (e.g. glycogen, starch, inulin).
According to their occurrence, polysaccharides can be divided into zoopolysaccharides (e.g. glycogen, chitin), phytopolysaccharides (e.g. cellulose, pectin substances) and polysaccharides of microorganisms (dextrans).
Carbohydrate metabolism[edit | edit source]
Carbohydrates enter the body in food. They serve as a necessary source of energy and are also a source for the biosynthesis of other non-sugar compounds. Most tissues of a living organism have at least a minimal consumption of glucose. The need for glucose in the brain and in erythrocytes is unavoidable.
Glucose[edit | edit source]
Sources of glucose[edit | edit source]
Glucose is the most important monosaccharide involved in cell metabolism. It is used both as a source of energy and as a starting material for the synthesis of glycogen and other metabolites.
Blood glucose[edit | edit source]
The uniqueness of glucose as an energy substrate lies in the fact that glucose:
- it is the only substance from which it is possible to obtain energy even in the absence of oxygen (hypoxia) and without mitochondria; precisely because of the absence of mitochondria, erythrocytes are dependent on glucose;
- it is a source of acetylCoA, as a substrate for the citrate cycle, for some tissues - e.g. the CNS
- it cannot be synthesized from fatty acids due to the irreversibility of the pyruvate dehydrogenase reaction. On the contrary, when there is an excess of glucose, fatty acids and subsequently triacylglycerols (TAG) can be synthesized from it.
For the above reasons, it is inevitable that a constant concentration of glucose in the blood - glycemia - is maintained in the body .
Determination of the concentration of glucose in the blood, i.e. glycemia, is one of the most common examinations in the clinical-biochemical laboratory. The average fasting blood glucose concentration of healthy people is 3.6–6.1 mmol/l. Elevated values can indicate the possibility of diabetes mellitus, acidosis, infection,
acute inflammation, CO poisoning, etc. Reduced values can be a manifestation of starvation, glycogenosis, arsenic, phosphorus poisoning, etc.
Selected methods of examination of glucose metabolism[edit | edit source]
- Blood glucose determination
- Determination of glycosuria
- Oral glucose tolerance test
- Determination of glycated hemoglobin
Glycogen[edit | edit source]
Glycogen is a storage form of carbohydrates for animals and humans. It is synthesized from glucose that has been ingested with food at a time when it was not needed as an energy source. When blood glucose levels drop, glucose is released from glycogen into the blood. The entire complex of reactions of glycogen synthesis and glycogen breakdown is catalyzed and regulated by the enzymes of glucose metabolism, glycogen synthesis and glycogen breakdown. The activities of these enzymes are in turn controlled by hormones – adrenaline, glucagon, insulin and allosteric effectors such as ATP , AMP, Glc-6-P and glucose. A comparison of the metabolism and function of glycogen in the liver and in the muscles is on the following pictures.
Disorders of glycogen metabolism are caused by a genetically determined lack of certain enzymes involved in this metabolism. Under pathological conditions, glycogen can be excessively stored in tissues (in the liver, heart and muscles), which impairs their function. Such diseases are called glycogenoses . The most serious are the forms in which the heart muscle is affected.
Glycoproteins[edit | edit source]
Distribution of glycoproteins[edit | edit source]
| Glycoprotein | Binding type | Type | Protein content |
|---|---|---|---|
| N-glykosides | -Asn-GluNAc-Man-… | blood plasma | 25–98% |
| O-glykosides | -Ser-Xyl-Gal-Gal-…uronates (O-SO3−) | proteoglycans | ~ 11% |
| -Ser(Thr)-GalNAc-Gal-… | mucin, blood plasma | 25–40% | |
| -Hyl-Gal-Glc-… | collagen | 89–99% |
Synthesis, transport and function of glykoproteins[edit | edit source]
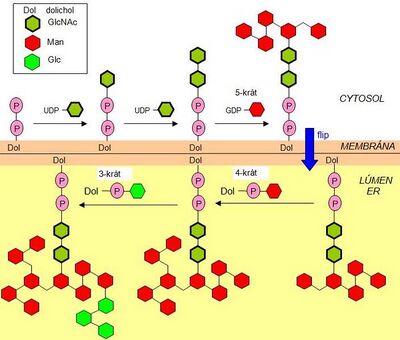
The first stage of oligosaccharide biosynthesis takes place in the cytosol and is completed in the lumen of the endoplasmic reticulum after the flip of dolichol-P with the bound oligosaccharide. The dolichol molecule is localized in the ER membrane.
Transport and function of glycoproteins:
- lysosomes (obr. 9) – hydrolases
- cytoplasmatic membrane – receptors, antigenic determinants, enzymes, structural and protective function
- extracellular space (exocytosis) – transport glycoproteins, enzymes
Clinically significant carbohydrates[edit | edit source]
Glucose
Examination of the level of glucose in the blood is not only among the most frequent examinations of carbohydrates in medical practice, but glucose is also one of the most frequently examined substances. It tells about the current state and proper regulation of carbohydrate metabolism.
The most common disease related to a violation of the regulation of glucose metabolism is diabetes mellitus . The cause of impaired glucose metabolism in diabetes is an insufficient amount of insulin to move glucose from the blood into the cells (1. low or no secretion of insulin by the pancreas, 2. sufficient insulin, but a malfunction of the insulin receptors). This results in:
- long-lasting elevated blood glucose level and with it associated:
- unwanted glycosylation of proteins and hemoglobin associated with organ damage (retina, kidneys, nerves, blood vessels, etc.)
- glycosuria (when the blood glucose concentration exceeds 10 mmol/l - poor resorption of glucose in the proximal tubule is not enough). Because glucose is osmotically active, its transfer into the urine causes excessive urination and dehydration.
- insufficient supply of glucose to the cells with the subsequent replacement use of fats as the main source of energy. Increased oxidation of fatty acids results in excessive formation of ketone bodies (acetone, acetoacetic acid, β-hydroxybutyric acid) associated with a drop in blood pH - ketoacidosis.
In addition to diabetes mellitus, examination of the blood glucose level is also used in the diagnosis of many pathological conditions that are associated with changes in carbohydrate (energy) metabolism (e.g. liver disease, hormonal disorders, some tumors, congenital metabolic disorders, poisoning).
Other monosaccharides
Defects in various enzymes of carbohydrate metabolism lead to congenital diseases. They are autosomal recessive (that is, one functional allele is enough to preserve the functionality of the enzyme) and are not frequent. The pathophysiology of these diseases can be derived from knowledge of biochemistry.
Fructose disorders of fructose metabolism can have varying medical severity. The following enzyme defects, which are inherited in an autosomal recessive manner, have been described:
- lack of fructokinase – essential fructosuria, in which increased excretion of fructose in the urine occurs after ingestion of fructose. It has no other manifestations.
- lack of fructose-1-phosphate aldolase – congenital intolerance of fructose, associated with the accumulation of fructose-1-phosphate. After the administration of fructose (even in the form of sucrose) it rises significantly in the blood, severe hypoglycemia occurs (convulsions and coma may also accompany it). Untreated, it has serious consequences on the central nervous system and can lead to death. People with this disorder must not consume anything containing fructose (sucrose, fruit, honey, etc.).
- lack of fructose-1,6-bisphosphatase – manifests itself after administration of fructose by hypoglycemia and lactic acidosis. The conversion of fructose-1-bisphosphate to fructose-6-phosphate is impaired, thereby blocking gluconeogenesis.
Galactose
After resorption, galactose is used for the synthesis of various substances (glycoproteins, glycolipids, proteoglycans, lactose) or is converted into glucose. Pathological conditions in which this conversion is disturbed and galactose or one of the intermediate products of galactose metabolism accumulates in the body, their concentration in the blood increases and passes into the urine are called galactosemia. Galactosemias are enzyme defects with autosomal recessive inheritance. The function of the following enzymes may be impaired:
- galactokinase – galactose is not phosphorylated on galactose-1-phosphate and accumulates in the blood. Within a few months after birth, it causes serious vision damage - cataract . With early diagnosis and exclusion of galactose (including breast milk ) from food, the damage can be prevented.
- galactose-1-phosphate-uridyltransferases – there is no conversion of galactose-1-phosphate + UDP-glucose to glucose-1-phosphate+ UDP-galactose. The result is the accumulation of galactose-1-phosphate, galactose and galactitol in various organs (eye, liver, kidney, heart, brain, intestine) and in erythrocytes, which is associated with their damage. If milk is not eliminated from the diet as soon as possible, the disorder of this enzyme manifests itself in mild forms of diarrhea and jaundice, in severe forms of severe hypoglycemia, diarrhea, mental retardation, blindness, metabolic disorders, severe liver damage and even death.
- 4-epimerase – the disorder of this enzyme has a benign clinical manifestation compared to the previous disorders. It is associated with a slightly increased level of galactose in the blood. The conversion of UDP-galactose to UDP-glucose is impaired.
Disaccharides
Under normal conditions, disaccharides from food are broken down into absorbable monosaccharides in the intestine. Disaccharidases found in enterocytes - lactase, maltase , isomaltase, sucrase - are responsible for this cleavage . A decrease in their activity is inherited in an autosomal recessive manner, and the number and activity of impaired enzymes can fluctuate. Either all disaccharides are missing and it is necessary to completely exclude disaccharides from the food, or the defect is isolated and only some disaccharide should be omitted from the food. Clinical manifestations of disaccharidases deficiency are usually osmotic diarrhea, abdominal discomfort (bloating, pain) and, in children, failure to thrive.
Glycogen
Glycogenoses – disorders of glycogen metabolism are again enzymatic disorders that are inherited in an autosomal recessive manner. Depending on whether it is due to a synthesis disorder or glycogen degradation as a result of damage to only one organ or several organs, it is divided into organ and generalized forms. With gradual discovery, the number of glycogenoses increases. Table 3.2 provides an overview of the most well-known types of glycogenases and their basic characteristics.
Non-enzymatic glycation of proteins
Glucose is capable to a certain extent of non-enzymatically binding to free amino groups of lysine to form a Schiff base. In this way, for example, < 6% of hemoglobin is glycated. This process is reversible and the glycation is directly proportional to blood glucose levels over the past month or so. Therefore, the % of glycated hemoglobin is determined in diabetics to assess the quality of their long-term compensation.
The consequences of non-enzymatic glycation of proteins are, for example, inactivation of enzymes, inhibition of the formation of regulatory molecules, cross-linking of glycoproteins, reduced sensitivity to proteolysis, abnormalities in function.
Tabulka 1 Poruchy metabolizmu glykogenu
| Type | Missing enzyme | Defect localization | Clinic | Therapy | |
|---|---|---|---|---|---|
| 0 aglycogenosis | Glycogen synthase | liver | Hypoglycemia, mental retardation | protein diet | |
| I Von Gierke | Glucose-6-phosphatase | liver, kidney, small intestine, leukocytes (Leu), platelets (Tr) | Enlarged liver and kidneys, hypoglycemia, metabolic disturbances, failure to thrive, altered distribution of abdominal and facial fat | Frequent supply of carbohydrates (except galactose and fructose), limit the supply of fats, protein diet | |
| II Pompe | Lysosomal α-1,4-glucosidase, α-1,6-glucosidase | all cells | Psychomotor retardation, lethargy, enlarged heart, death | Megadoses of vitamin A, glucosidase supplementation, bone marrow transplants | |
| III Forbes | Amylo-1,6-glucosidase | liver, muscles, heart, erythrocytes (Ery), Leu | Mildly enlarged liver and heart, muscle involvement. | Frequent feeding, protein diet | |
| IV Andersen | 1,4-α-glukan-6-α-glukosyltransferase | liver, heart, muscles, Leu | Enlargement of liver and spleen, emaciation, death from enlarged liver and heart | Liver transplantation | |
| V McArdle | Phosphorylase | muscles | Weakness, fatigue, twitching, convulsions, muscle breakdown - rhabdomyolysi | Consumption of glucose and fructose, limit exertion | |
| VI Hers | Phosphorylase | liver | Enlargement of the liver | Frequent feeding, protein diet | |
| VII Tarui | Phosphoglucomutase | muscles, sometimes liver | Weakness | According to the symptoms | |
| VIII | Phosphohexose isomerase | liver?, brain? | Liver enlargement, CNS damage, ``dancing eye symptoms'' (nystagmus), tremors, muscle tone disorders, death | ||
| IX | Phosphorylase kinase | IXa – liver, IXc – liver, muscles, IXb liver – X- heredity | Enlargement of the liver and spleen, mild muscle damage, the condition normalizes with age | Unnecessary | |
| X | cAMP-dependent kinase | liver, muscles | Liver enlargement, muscle pain, convulsions | Unknown | |
| XI | Phosphoglucomutase | liver, kidneys |
|
Administration of phosphates |
Links[edit | edit source]
Related articles[edit | edit source]
- Carbohydrates in Human Nutrition
- Lipids (1. LF UK, NT) • Lipids in Human Nutrition • Lipids as an energy source • Fatty acids
- Proteins in the diet • Amino Acids
- Diabetes Mellitus • Glycogenosis