Intracranial hypertension
Intracranial hypertension is defined as an increase in intracranial pressure (ICP) above 20 mm Hg. Due to the strength of the calva, it can occur when any of the 3 basic intracranial compartments – brain tissue, cerebrospinal fluid and blood volume are enlarged.
Physiology and pathophysiology of intracranial pressure[edit | edit source]
The brain represents only 2 % of body mass, but cerebral perfusion represents 15 % of resting cardiac output, 20 % of O2 consumption. The brain is inherently an expansive structure and expands with each heartbeat. Because no valves are present during venous outflow from the brain, the change in intrathoracic pressure is reflected in the ICP value. A phenomenon that proves this statement is the pulsating large fontanelle even in some completely healthy children. The above-mentioned close relationship of intracranial pressure to intracranial pressure is the reason why we set PEEP values as low as possible during artificial lung ventilation in patients with intracranial hypertension.
In an adult, the normal mean value of ICP is around 15 mm Hg, with a wider range of 2-20 mm Hg. For newborns, we tolerate values < 10 mm Hg. The curve fluctuates slightly depending on the cardiac cycle.
The relationship between the volume of the skull and ICP is described by the Monro-Kellie doctrine – the cranial cavity has a fixed volume filled with three compartments - brain tissue, cerebrospinal fluid and blood, which are practically incompressible (due to the high proportion of water in brain tissue).
An increase in one of the compartments can be balanced by compensatory mechanisms: the movement of cerebrospinal fluid from the cranial cavity to the spinal canal, a decrease in the volume of blood in the skull (mainly in the veins) and certain elastic brain tissue. If the changes are slow, the elasticity and remodeling of the brain tissue will allow considerable compensation, for example in normotensive hydrocephalus. After exhausting these mechanisms, when one of the components increases ICP exponentially. The beginning of the failure of compensatory mechanisms can be observed during ICP monitoring, for example, by a transient increase in ICP, for example, during coughing or in response to suctioning from the airways, which should be a warning signal for the development of intracranial hypertension.
Cerebral perfusion pressure[edit | edit source]
Cerebral perfusion pressure (CPP) is defined as the difference between arterial and venous pressure in the cerebral vessels. The magnitude of this pressure is essential to maintaining a good blood supply to the brain. Since venous pressure is difficult to monitor and is practically close to intracranial pressure, it is used to calculate the following equations:
- CPP – cerebral perfusion pressure
- MAP – mean arterial pressure (measured invasively, e.g. in the radial artery)
- ICP – intracranial pressure
From this equation, it follows that during the therapy of intracranial hypertension, in addition to monitoring one's own ICP, it is also necessary to maintain sufficient arterial pressure to maintain normal cerebral perfusion.
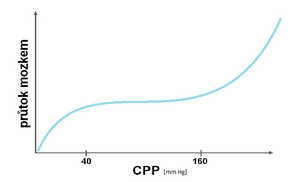
Autoregulation of cerebral blood flow[edit | edit source]
This property prevents the occurrence of ischemia or cerebral edema by providing relatively (CBF – cerebral blood flow) when the CPP changes in the regulatory lumen of the cerebral vessels. Physiologically, flow is stable in the CPP range of 40–160 mm Hg. Changes in flow depending on CPP are shown in the pressure-flow curve.
Cerebral Metabolic Oxygen Consumption (CMRO2)[edit | edit source]
Brain Metabolic Oxygen Consumption, CMRO2 (cerebral metabolic rate of O2):
Failed to parse (syntax error): {\displaystyle CMRO_{2}=CBF×AVDO_{2}}
- AVDO2 = cerebral arteriovenous difference
- CBF = Cerebral Blood Flow
Clinicalsituations with increased metabolic activity such as convulsions or fever will be manifested by an increase in CMRO2, usually compensated by increased cerebral blood flow. Hypothermia reduce oxygen consumption. Conclusions for treatment options are then based on this assumption, i.e. reduction of the level of cerebral metabolism of sedation, antipyretics, antiepileptics so that the balance between CRMO2 and CBF is maintained. This is also a theoretical assumption for the so-called therapeutic barbiturate coma.
Cushing's reflex[edit | edit source]
- With intracranial hypertension, we detect a rise in blood pressure as a reflex effort to maintain cerebral perfusion (CPP);
- in addition, bradycardia will arise from irritation of the nervus vagus;
- if breathing disorders increase, we are talking about Cushing's trias.
Causes of intracranial hypertension[edit | edit source]
Based on the Monroe-Kelly doctrine, the cause can be an increase in one of three compartments - brain tissue, amount of cerebrospinal fluid and blood in the forehead. The causes can influence each other – the expansive process of the brain tissue can lead to obstructive hydrocephalus by obstructing the cerebrospinal fluid pathways or vasogenic edema by breaking the blood-brain barrier. Vasogenic edema can be complicated by cytotoxic edema, which can also be a consequence of ischemia due to various causes.
Changes in brain tissue[edit | edit source]
The cause of intracranial hypertension on the part of the brain tissue can be, for example:
- brain tumors,
- brain abscess,
- arachnoid cyst,
- a cyst of another origin, for example echinococcal,
- brain oedema – an increase in water in the brain tissue, it has several types:
- cytotoxic edema – with hypoxia of brain cells, most often after an injury or stroke;
- vasogenic edema – in case of damage to the HEB, most often in the case of a tumor or infection;
- interstitial edema – in case of obstruction of CSF outflow;
- hypoosmolar edema – in disorders of mineral metabolism.
CSF changes[edit | edit source]
80 % of CSF production takes place in plexus choroideus, the rest is produced by endothelial cells. It is an active process dependent on energy and the appropriate enzyme equipment. CSF is distributed to the cerebrospinal fluid and subarachnoid spaces. The CSF spaces are formed by two lateral chambers, the third chamber, the aqueduct, the fourth chamber and the spinal central canal. Resorption takes place through the arachnoid villi into the superior sagittal sinus. It is conditioned by the pressure gradient between brain pressure and venous blood pressure in the sagittal sinus. Another possibility is resorption in the area of the spinal canal into the venous plexus in the sheaths of the spinal roots. A small portion of the CSF drains through the olfactory bulb and mucosa into the deep neck nodes.
An increased amount of cerebrospinal fluid is present in acute hydrocephalus, especially obstructive hydrocephalus (e.g., when compressed by a tumor or occlusion of a coagulum after a subarachnoid hemorrhage). The aforementioned arachnoid cyst is also filled with cerebrospinal fluid.
Changes in blood volume[edit | edit source]
An increased amount of blood in the intracranial space is present in all types of intracranial hemorrhage (subarachnoid hemorrhage, subdural hemorrhage, epidural hemorrhage, hCMP) and cerebral hyperemia (brain swelling). In cerebral hyperemia, there is a loss of autoregulation due to damage to the hypothalamus and brainstem, which leads to an increase in flow through the cerebral vessels, an increase in the volume of blood in the brain, which raises the ICP and, as a result, leads to obstruction of the venous outflow and thus a decrease in CPP and cerebral ischemia. This condition is more common in children.
Clinical symptomatology and diagnostics[edit | edit source]
In the anamnesis, we ask about previous injury, possible intoxication, present convulsions, breathing disorders, amount and frequency of urination, fever, visual disturbances.
Prodromal symptoms of intracranial hypertension include:
- headaches;
- vomiting/nausea;
- unrest;
- apathy or irritability;
- for children then further:
- irritable crying (in infants, a typical "wailing" and high-pitched cry);
- the large fontanelle rises;
- extension of the venous plexus on the skin of the head.
Initially, we register tachycardia, tachypnea and fluctuations in systemic pressure. During progression, disturbances of consciousness, loss of trunk reflexes, tone disorders, convulsions occur.
Typical manifest symptoms of intracranial hypertension are:
- pathological reaction of the pupils (initially miosis with hyperventilation and hyperpnea, with massive brain edema, then mydriasis with lesions of nuclei of n. III, anisocoria);
- hyperventilation;
- often a combination of hypertension with bradycardia (along with Cushing's triad respiratory disorders);
- changes in heart and respiratory rate during head flexion;
- disorder of consciousness.
Clinical symptoms at a critical ICP value are hypertension, bradycardia and bradypnea ending in apnea.
A clinical examination in pediatrics includes a complete pediatric examination, including a tap on the calf (= sign of a cracked pot). As part of the neurological examination, it is necessary to evaluate the state of consciousness (GCS), musculoskeletal reflexes on DK, examination of the brainstem (cranial nerves), condition of the pupils (mydriasis, anisocoria), mobility of the bulbs (paresis, diplopia), evaluation of momentum in the area of the facial nerve.
The brainstem reflexes include the nasopalpepbral reflex (blinking when the bridge of the nose is tapped) and the corneal reflex (blinking when the cornea is irritated). We finish the neurological examination with an examination of meningeal signs.
The basic and emergent examination for intracranial hypertension is CT of the head (we perform the examination only after securing and stabilizing the patient). A sign of cerebral edema on a CT scan is the effacement of gyrification, narrowing of the cerebral ventricles, and a decrease in the difference between the white and gray matter of the brain. An alarming symptom is the disappearance of the subarachnoid spaces, unfortunately this is already a late symptom. The EEG is usually postponed until after the CT examination due to the time requirement. Transcranial Doppler ultrasonography can also be used, and in infants, ultrasound through the "window" of the large fontanel.
With a unilaterally acting process, the displacement of the brain mass and the brain herniation can occur.
Intracranial pressure (ICP) monitoring[edit | edit source]
Nowadays, pressure sensors inserted directly into the tissues are used to monitor intracranial pressure. All methods are invasive with a risk of infection. All types of sensors are inserted by a neurosurgeon.
The gold standard in ICP monitoring is ventriculostomy – an intraventricularly introduced catheter that, in addition to accurate measurement of intracranial pressure, also allows pressure to be regulated by releasing cerebrospinal fluid.
Currently, intraparenchymal monitoring of intracranial pressure is most widely used (thin, introduced into the brain tissue with a small bore; high accuracy, simple installation). The pressure sensor is introduced from the hole above the non-dominant hemisphere (1 cm in front of the coronary suture) and in the sagittal plane interspersed with the pupil of the opposite side.
For supplementation within neurointensive care, we can use so-called multimodal focus monitoring, where temperature and pO2, pCO2 and pH sensors or microdialysis catheters can be introduced intracranially. Furthermore, monitoring of jugular blood oxygen saturation (SvjO2) is often used as part of multimodal monitoring, which makes it possible to determine the arteriovenous difference in cerebral circulation.
Indication of ICP measurement[edit | edit source]
- Brain trauma:
- GCS ≤ 8 and abnormal CT (hematoma, brain contusion, brain edema, compression of basal cisterns),
- GCS ≤ 8 with normal CT in the presence of at least two of the following conditions:
- age > 40 years,
- momentum disorder
- systolic BP < 90 mm Hg.
- monitoring after decompressive craniectomy (debatable).
- Non-traumatic brain diseases:
- indicated less often,
- after subarachnoid hemorrhage due to the risk of obstructive hydrocephalus via ventriculostomy,
- after evacuation of intracerebral bleeding after aneurysm rupture (part of the decision-making process for decompressive craniectomy),
- in congenital hydrocephalus.
- Other indications:
- criteria vary, for example general risk of increased ICP or suspected intracranial hypertension, diagnosis of intracranial process, some sources report indeterminate coma with GCS < 8.
Contraindications to ICP measurement[edit | edit source]
Infaust conditions, coagulation disorders, defunct ventricular system in the case of ventriculostomy.
Complications of ICP measurement[edit | edit source]
The risk of infectious complications increases significantly with the time of insertion of the sensor, it also increases with the frequency of evacuation of the cerebrospinal fluid during ventriculostomy. The risks of hemorrhage are 1.1 % with ventriculostomy, 2.8 % with intraparenchymal monitoring. Ventriculostomy obstruction occurs in 6.3 %. Sensor malfunction is very common, associated for example with inappropriate handling of the patient, its frequency is about 20%. ICP measurement also increases the risk of epilepsy, which is reported to be around 2-3 % with an intraparenchymal sensor. [1]
Therapy of intracranial hypertension[edit | edit source]
In addition to therapy for the primary disease, supportive therapy is used to reduce the risk of secondary brain tissue damage by increasing intracranial pressure. A manifestation of cerebral palsy is the clinical picture of a disorder of consciousness in an objective finding. Its severity is then guided by the indication for therapy.
The principles of care and monitoring are classified by some into graded protocols according to the manifestations of the severity of the disability. However, the classification of some therapeutic procedures varies in the literature.
Indications for intubation and artificial lung ventilation are:
- GCS value < 8 p. even during spontaneous breathing;
- disappearance of protective laryngeal reflexes;
- ventilatory insufficiency or spontaneous hyperventilation inducing hypoxic vasoconstriction in the CNS (pCO2 < 3,5 kPa);
- deepening of impaired consciousness by ∆GCS > 2 p.;
- bilateral jaw fracture;
- massive bleeding in the mouth and convulsions.
Glasgow Coma Scale and Score is the basis for determining the impairment of consciousness.
1st degree scheme[edit | edit source]
- Proper positioning of the patient with elevation of the upper half of the body – head position 15–30°, i.e. avoid constriction of the jugular veins.
- Insert an intraparenchymal sensor into the CNS to measure ICP, secure an arterial line for direct blood pressure measurement, and secure a central venous catheter, preferably via the subclavian vein, to measure CVP. Under these conditions, we can calculate the cerebral perfusion pressure (CPP)..
- The goal should be to maintain ICP < 20 torr, in young children < 15 torr, in neonates and infants < 10 torr and CPP > 50 torr, in neonates > 40 torr (CPP < 40 torr is critical). A disproportionately high CPP value (> 150 torr) can lead to the progression of vasogenic edema.
- Initial infusion therapy – so that the circulation is stabilized. Crystalloids 1/1 FR or 1/1 Ringer (NOT 1/1 Hartmann, as it is slightly hypoosmolar) are used, preferably hydroxyethyl starch from colloids. Initially, we administer the smallest necessary amount of fluids to achieve and maintain appropriate MAP values.
- UPV – according to intracranial pressure values. As a rule, it is sufficient to achieve capnia levels at the lower limit of the norm (it has a protective effect), normoxemia and SaO2 97 %.
- Maintain normothermia.
- Monitoring saturation in the jugular bulb in the first step phase is not a condition, but it can contribute already in the early phase of therapy to the identification of prognostically more serious cases (e.g. with low oxygen extraction, with an increase in the concentration of lactate in the blood taken from the jugular bulb). We keep the SvjO2 value > 60 %.
- Adequate extracranial homeostasis must also be maintained - i.e. maintain balanced water balance and normotension (or mild hypertension), blood glucose should be between 6-10 mmol/l, Na 145-150 mmol/l, serum osmolality 295-305 mmol/l.
- Continuous analgosedation (fentanyl, sufentanyl, propofol), because the patient's restlessness, cough, interference with the ventilator - all this can lead to an increase in ICP.
- Propofol as an anesthetic has an advantage in the initial phase of therapy due to its short half-life, rapid onset and the possibility of frequent re-evaluations of neurological findings.
- Myorelaxants can also be considered.
- Mannitol – creates a gradient for the outward movement of water from the tissue into the vascular bed, reduces blood viscosity and induces reflex vasoconstriction;
- the indication is particularly a sharp rise in ICP and a sudden worsening of the neurological findings;
- the effect is felt in 2 minutes, the greatest decrease in 20-60 minutes;
- its administration can be complicated by the rebound phenomenon during extravasation after damage to cerebral vessels, for example in the context of vasogenic edema.
- Hypertonic NaCl is used as a bolus for similar indications as Mannitol. Its effect on ICP correction seems to be better in the short term, but the long-term results have not been sufficiently analyzed.
- Furosemide – reduces ICP by reducing edema, synergizes with mannitol, suppresses CSF secretion;
- Fluid restriction – only in hypoosmotic edema.
- Corticosteroids – only in limited indications, especially for vasogenic edema and intracranial hypertension associated with tumor or brain infection;
- considered an essential option for spinal cord trauma therapy, but the evidence for their effectiveness is questionable, some meta-analyses also mention a preponderance of adverse effects over an uncertain positive benefit;
- corticoids should not be used in head trauma, intracranial hemorrhage, or ischemic stroke.
- If there is a higher risk of developing peptic ulcers (e.g. administration of non-steroidal anti-inflammatory drugs together with glucocorticoids), we administer antacids and H2 receptor blockers as prevention.
2nd degree scheme[edit | edit source]
- Hyperventilation causes vasoconstriction of cerebral vessels (decrease in CO2 partial pressure reduces ICP).
We increase alveolar ventilation so that pCO2 values of 4.0–4.5 kPa are reached when venous blood saturation in the jugular bulb is > 55 %.. The use of hyperventilation alone without the possibility of SvjO2 monitoring is risky – there is a risk of brain ischemia due to excessive vasoconstriction.
3rd degree scheme[edit | edit source]
- Thiopental will significantly reduce the metabolic demands of the CNS in the so-called barbiturate coma and thus reduce the consumption of O2 by the brain tissue. Thiopental reduces CMRO2, i.e. cerebral metabolism (by up to 50 %), leading to a decrease in cerebral blood flow. Among its other effects is a reduction in the levels of excitatory amino acids in the CNS (glutamate), it also has a stabilizing effect on cell membranes.
- Hypothermia decreases ICP by a dual mechanism. There is a decrease in CMRO2, resulting in a decrease in CBF and CBV (affecting vasogenic edema). The second mechanism is the stabilizing effect of hypothermia on cell membranes (influence of cytotoxic edema). The ideal temperature is 33–34 °C. We use local hypothermia (covering the head with ice) or general hypothermia. Hypothermia inhibits the function of neutrophils and therefore the risk of infection is increased. Hypothermia is indicated after failure of conventional therapy for intracranial hypertension.
- Ventriculostomy drainage of cerebrospinal fluid will help reduce ICP by shrinking the CSF compartment.
- Decompressive craniotomy may be the last resort to reduce intracranial hypertension. The anesthesiologist together with the neurosurgeon decides on the indication for the procedure in this phase. The method is suitable for patients who meet some or all of the following criteria:
- diffuse cerebral edema on CT;
- time < 48 hours since the insult;
- no long episodes of ICP > 40 torr;
- GCS > 3 points;
- secondary deterioration of the clinical condition;
- development of symptoms of cerebral herniation unresponsive to mannitol.
Evaluation of treatment results[edit | edit source]
Treatment results are evaluated by the so-called Glasgow Outcome Scale (GOS) after 6 months:
- survival with good outcome = able to live independently with minimal or no neurological impairment;
- moderate impairment of brain functions = neurological and intellectual disability with the possibility of independent living;
- severe cerebral impairment = conscious but completely dependent on assistance with daily activities;
- vegetative state;
- death.
Links[edit | edit source]
Related articles[edit | edit source]
- Intracranial hypertension/ PGS
- Brain oedema
- Optic Disc Swelling
- Hemodynamic swelling of the brain
- Brainstem reflexes
External links[edit | edit source]
- Intracranial Hypertension and ECG - Free ECG book
- Monitoring of patients with severe brain injury Martin Smrčka 2011
- Template:Mefanet
- Template:Mefanet
References[edit | edit source]
- ↑ ŠEVČÍK, Pavel, et al. Intenzivní medicína. 3. edition. Galén, 2014. 1195 pp. pp. 459–465. ISBN 9788074920660.
Bibliography[edit | edit source]
- SMITH, Edward R. Evaluation and management of elevated intracranial pressure in adults [online]. UpToDate, The last revision 2020-04-26, [cit. 2020-05-14]. <https://www.uptodate.com/contents/evaluation-and-management-of-elevated-intracranial-pressure-in-adults>.
- ZEMAN, Miroslav, et al. Speciální chirurgie. 2. edition. Praha : Galén, 2004. 575 pp. ISBN 80-7262-260-9.
- NEVŠÍMALOVÁ, Soňa – RŮŽIČKA, Evžen – TICHÝ, Jiří. Neurologie. 1. edition. Praha : Galén, 2005. 163-170 pp. ISBN 80-7262-160-2.
- AMBLER, Zdeněk. Základy neurologie. 6. edition. Praha : Galén, 2006. pp. 171-181. ISBN 80-7262-433-4.
- SAMEŠ, M, et al. Neurochirurgie. 1. edition. Praha : Jessenius Maxdorf, 2005. ISBN 80-7345-072-0.
- HAVRÁNEK, Jiří: Intrakraniální hypertenze.
- Otázky J. Beneše, zdroj: přednášky