Electrical and magnetic properties of tissues
Tissues and organs exhibit electrical properties that we divide into passive and active:
passive electrical properties' - behavior of tissues in an electric field (conductivity, capacity),
active electrical properties' - electrical manifestations associated with the tissue's own activity.
Passive electrical properties[edit | edit source]
Tissues behave as a special kind of conductor in an electric field. Compared to metal conductors, they show significant macroscopic and microscopic 'inhomogeneity' caused by their anatomical, histological and molecular structure. Electric current passes through environments of different viscosity, structure and chemical composition, therefore' tissue conductivity depends on the specific type of tissue'.
| Tissue | Resistivity (Ω.m) |
|---|---|
| cytoplasm of a cell | 1 |
| body fluids | 0.8–1.3 |
| muscles | 3 |
| parenchymatous tissue | 4–6 |
| adipose tissue | 10–15 |
| bone tissue | 30 |
Active electrical properties[edit | edit source]
The basis of active electrical properties of tissues is membrane potential cell. The distribution of ions is different intracellularly and extracellularly and creates the so-called resting membrane potential', which is characteristic of every living cell. Its value ranges from -30 to -90 mV, so the inside of the cell is negatively charged compared to the extracellular space.
In addition to resting membrane potential, we also talk about action potential. The latter is characteristic of only two types of cells – nerve and muscle. We call tissues formed by such cells excitable'.
In principle, it is a change in the resting potential, caused by the modulation of the permeability of the membrane for sodium cations, which flow into the cell after a drop in concentration. This change starts a cascade of cellular events, leading to the realization of the function of the given tissue:
- nervous tissue is adapted to the rapid conduction of impulses over long distances,
- muscle tissue converts the energy of chemical bonds into mechanical work.
For the proper functioning of the system and the possibility of re-creating the action potential, it is important to return to the resting membrane potential, in which the transport systems in the cell membranes participate.
The electrical manifestations of 'whole organs and tissues' are much more complex compared to simple action potentials and are the result of 'interference' and temporal and spatial summation of action potentials' of individual cells, influenced by passive electrical properties of tissues.
The measurement of action potentials in tissues is the basis of the biophysical disciplines electrocardiography, 'electroencephalography and '[[electromyography] ]. It is also used for ``electrodiagnosis of tissue and organ disorders.
Magnetic properties of tissues[edit | edit source]
According to the theory of the electromagnetic field, when electric charges move, an induced magnetic field is created. It is therefore obvious that a similar process will also occur during the electrical activity of living systems as a result of local ion currents.
Detection of such a small magnetic field was not possible until the first half of the 1960s, when the highly sensitive 'superconducting quantum magnetometer (SQUID)' was constructed. This is also an obstacle, as it captures a large number of interfering magnetic fields that do not come from the object under investigation.
The detection of magnetic fields arising in living systems is therefore still difficult and requires special conditions and shielded spaces.
Individual tissues have a different biochemical structure, i.e. different representation of protons, outwardly they are manifested by different magnitudes of magnetic moments and thus inform about their composition.
An atomic nucleus consists of protons and neutrons. Protons constantly rotate around their own axis, or perform a conical, circular motion - 'precession (spin). Every charged particle that moves creates a magnetic field around it. Each proton behaves like a compass needle. If it is brought into another magnetic field, it orients itself in the direction of its action, only rarely the other way around.
Atomic nuclei with an even nucleon number do not behave magnetically to their surroundings because their magnetic moments cancel and cannot be used for magnetic resonance imaging. Atomic nuclei with an odd nucleon'' number retain their magnetic moment, for example 1H, 13C, 19F, 23Na, 31P. Since the human body is made up of more than 60% water, hydrogen is the most suitable object for magnetic resonance (MR) imaging. All tissues containing hydrogen, proteins, sugars, fats and other macromolecular substances of the human body can be used for MR imaging.
Under normal circumstances, the orientation of the magnetic poles of the protons is random and they cancel each other out, so the tissue looks non-magnetic on the outside. However, if we place the tissue in a strong magnetic field, the axes of the protons will be arranged parallel to the lines of force of the external magnetic field. Most of them are oriented parallel to the vector of the external magnetic field, a smaller number of protons is more energy demanding. This phenomenon causes the tissue to exhibit a total magnetic moment and behave externally magnetically. This property is the basic principle of magnetic resonance.
Magnetic signals are sensed ``non-contact, which excludes artifacts caused by transient resistances between the electrode and the tissue during contact sensing of bioelectrical voltages.
Nuclear Magnetic Resonance[edit | edit source]
The object for our own application is the hydrogen protons found in our organism. Since protons show the energy equipment of a small magnetic field, after being placed in another magnetic field, they orient themselves in the direction of its action, exceptionally the other way around. Protons are then high-frequency waves with a specific frequency deviated from their equilibrium state. By receiving this energy, the protons reach a state of saturation. The prerequisite for the effect of the resonance signal is the induction frequency corresponding to the energy difference of both proton positions (resonance). During the subsequent relaxation process, the protons release the absorbed energy and return to their original position with respect to the magnetic field.
The rate of relaxation is determined by 'tissue constants. T1- and T2- relaxation times are different depending on the type of tissue and provide better contrast in imaging than CT or X-ray images. MR imaging corresponds to the topography of the MR signal, expressed in different degrees of gray. The MR signal is dependent on the properties of the magnetic field, the imaging technique used, or the pulse sequence of the magnetic field. It also depends on the characteristics of the examined tissues, including the method used to record T1 and T2 relaxation times, on the density of free protons and other factors, such as the ratio of magnetization to the intensity of the magnetic field, chemical shift (excitation), blood flow.
MR images can be so-called T1, T2 or proton "balanced". The first of the methods controls the image while monitoring the relaxation in the longitudinal direction of the fundamental magnetic field. The second method is current in the transverse plane to the static magnetic field. A clearer contrast must then be achieved by spatial electromagnetic manipulations. The whole process is further dependent on the selected sequence of magnetic pulses, the repetitive time TR (interval between the repetition of pulse pulses), and the echo time (interval between the future pulse and MR signal measurement).
In order to create an image from the MR signal, the place of its origin must be localized. This is possible after 'spatial analysis of the investigated object, so that the reconstruction of the image of a certain layer is possible. The same will happen with the gradients linearly in space of the increasing magnetic field, which will be superimposed in three planes by the main homogeneous magnetic field. Signal noise is essentially dependent on the strength of the main magnetic field. Devices with a magnetic field strength of 1.0–1.5 Tesla are suitable for creating a high-quality, sharp image. The local result then depends on the selected field of view (FOV) and also on the thickness (thickness) of the layer.
Electrical properties of muscles[edit | edit source]
Characteristics of Skeletal Muscle[edit | edit source]
Skeletal (striated muscles) are made up of 'muscle bundles'. These consist of muscle fibers that arise from the fusion of precursor cells (myoblasts). They are therefore syncytial formations (syncytia = multinucleated cells that are formed by the fusion of several cells). Muscle fibers contain a large number of 'myofibrils ' - part of the cellular skeleton - which are made up of thin (actin) and thick (myosin) myofilaments '. Myofilaments are fibrous protein complexes. Myofibrils are capable of contraction and are divided into basic functional units – sarcomeres'.
Transverse striations are formed by the partial overlap of actin and myosin filaments in the sarcomere.
 The actin filament is formed by a double helix of the filamentous polymer F-actin, which consists of globular units of G-actin. Molecules of the fibrous protein tropomyosin are attached to the gap between the helices, and molecules of the protein troponin are attached to it. Troponin is very important for muscle contraction. It has three subunits that have different functions:
The actin filament is formed by a double helix of the filamentous polymer F-actin, which consists of globular units of G-actin. Molecules of the fibrous protein tropomyosin are attached to the gap between the helices, and molecules of the protein troponin are attached to it. Troponin is very important for muscle contraction. It has three subunits that have different functions:
- Tn-C: binds Ca2+ ions if available.
- Tn-T: links troponin to tropomyosin.
- Tn-I: changes the position of tropomyosin and thus exposes binding sites for myosin.
myosin filament Myosin is a motor protein (capable of movement). A myosin filament is made up of two molecules that have a "golf club" shape and wrap around each other. Myosin filaments polymerize and the resulting polymer is up to 1.6 μm in length. In its long part, it is formed by a rod-shaped polypeptide double helix of myosin chains and at the ends of the molecules by globular myosin, where there is a site for binding actin.
Characteristics of smooth muscle[edit | edit source]
The basis is a spindle-shaped smooth muscle cell, about 20 μm long. Myofibrils are not formed in the cell, but myofilaments are - again thin and thick.
The thin myofilament has a similar structure and composition to skeletal muscle, but the troponin complex is missing, so the binding sites for myosin are still exposed. In smooth muscle, only a maximum of ten myosin molecules make up the polymer of the thick myofilament.

Muscle contraction[edit | edit source]
The principle of muscle contraction depends on the spontaneous binding of actin and myosin and the ability of myosin to move along microfilaments (even elsewhere than in muscles, e.g. during the transport of vesicles in the cell). thick myofilaments. There is a shortening - contraction of the sarcomere.

The condition for achieving muscle contraction is a sufficient concentration of "calcium cations Ca2+" and a sufficient concentration of "ATP molecules" (energy)
The 'Mechanism of muscle contraction begins with the neuromuscular action potential (AP) transmission - excitation. The impulse (AP) arrives at the nerve ending and causes the exocytosis of vesicles with the mediator - acetylcholine (ACh). ACh binds to nicotinic receptors on the sarcolemma and this binding causes chemically gated Na+ channels to open. Thanks to the concentration gradient, sodium can thus diffuse into the cell and cause local depolarization (plate potential) - reducing the polarization of the cell membrane. A coupling occurs between excitation and contraction. Even if there are enough ATP molecules in the sarcoplasm for contraction to occur, it is first necessary to link actin to myosin. This requires an increase in the limiting concentration of calcium cations''. Thanks to the propagation of AP along the muscle fiber (with the help of Na+ and K+ ions) the channels for Ca2+ (voltage controlled) are opened. Calcium cations are thus released from the sarcoplasmic reticulum in the sarcolemma. Calcium binds to Tn-C, Tn-I changes shape and allows tropomyosin to fit into the groove, exposing actin binding sites for myosin. In this way, myosin heads and actin molecules are immediately bound. When actin and myosin are already bound to each other, 'splitting of ATP molecules can occur. The energy released from this splitting is used to bend the heads ("golf club ends") and thus pull the set of thin myofilaments between the thick myofilaments. The reaction products (ADP + phosphate) leave the head and make room for another ATP molecule. When it binds, actin and myosin are disconnected and myosin moves on the thin myofilament one globular actin away. The whole cycle can keep repeating itself. This is how the myosin moves the actin filaments and, thanks to this, the contraction of the muscle increases.
'Relaxation occurs when calcium cations are sucked out of the sarcoplasm back into the sarcoplasmic reticulum, tropomyosin is pulled out of the groove of the thin myofilament by troponin I - disconnection of actin from myosin.
Electrical properties of muscles[edit | edit source]
Electrical biosignals are the result of electrochemical processes that take place inside the cells and especially between the cells of the body. The total action potential is represented by the flow of ions through the cell membrane. Action potentials of excited cells are transmitted to adjacent cells and can create an electric field in the corresponding biological tissue. A weak electric current thus runs through the muscle in the direction in which ions spread from the nerves to the peripheral parts of the muscles.
Using the electrical properties of muscles[edit | edit source]
EMG - electromyography[edit | edit source]
Electromyography is a recording of electrical activity occurring in skeletal muscle. The goal is to investigate muscle action potentials - potentials created as a result of skeletal muscle activity. Surface electrodes cannot be used because they would sum signals from too many muscle fibers. Therefore, injection electrodes are used, which register the signal from only a small number of motor units. The amplitude of the measured signal reaches 50 μV to 1 mV. Diagnostically interesting events have a frequency of 10 Hz to 3 kHz.
Therapeutic uses of direct current[edit | edit source]
Direct current (galvanic current) passes mainly through the extracellular fluid, the tissue takes place as the movement of ions. When switched on and off (does not irritate at a constant intensity, but can change the irritation). Electrostimulation methods that use direct current:
Galvanization is applied in an aquatic environment. When the current passes through the tissues, its complete polarization occurs, which the organism evaluates as a serious disorder and reacts by maximally increasing capillary blood flow. It is used, for example, to treat post-traumatic conditions, tendinitis, muscle pain, nerve problems, degenerative diseases of the musculoskeletal system and conditions after paralysis.
'Iontophoresis' uses a galvanic current to apply drugs in the form of ions to the body. Magnesium, mesocaine, hyaluronidase are applied from the anode; from the cathode bromine, iodine, salicylic acid, ascorbic acid. Medicines are applied with the help of iontophoresis to numb the skin, some skin diseases, to improve blood circulation in the limb, to soften fibrous tissue.
Medicinal uses of alternating current[edit | edit source]
"Alternating current" is also used therapeutically. A large amount of heat is generated during its passage. Its therapeutic effect depends on many factors: frequency, amplitude, shape and modulation of impulses, type of tissue. Low-frequency currents (with a frequency of up to 100 Hz) have irritating effects, high-frequency currents (with a frequency above 100 Hz) have thermal effects. Trabert currentslow-frequency, monophasic currents are used for pain in the neck, back, head and limbs, they have an analgesic effect.
Diadynamic currents' are the simultaneous application of direct current and pulsed current. Depending on the degree of intensity, they have different effects. At above-threshold sensitive intensity, they have an analgesic effect, at below-threshold motor intensities they cause muscle contractions (myostimulation, myorelaxation).
Transocular electrostimulation (TENS): uses point applications of electrical impulses (low-energy, frequency 80-120 Hz) suspended by a blunt needle electrode. There is a release of endorphins, immediate suppression of pain. Use: painful conditions of the locomotor system, maintenance of muscle tension of injured or temporarily denervated muscles, prevention of muscle atrophy (in case of fractures), phantom pain.
With ``interference currents, two medium-frequency currents of unequal frequencies (around 5000 Hz) interact with each other — the resulting frequency is determined by the difference between the two, i.e. about 100 Hz. Lower frequencies (5-20 Hz) have an irritating effect, they tone the nervous system. Higher frequency (50-100 Hz) has a dampening, analgesic effect, relaxes muscle contractions.
Diathermy' enables deep heating of tissues by converting the energy of the high-frequency electromagnetic field into the internal energy of the tissues. The shorter the wavelength, the more intense the tissue heating.
- Shortwave diathermy uses high frequencies that do not require a conductive connection between the electrodes and the organism, the heated tissue is a dielectric. Application: by condenser field (overheating mainly in the subcutaneous tissue → diathermy of the abdomen, joints, chest or parts of the limbs); by the induction field of the spiral (heating in the electromagnetic field → acts on the muscles); pulse application (local heating occurs).
- "Ultra short wave diathermy (ultrasound - US)" uses the biological effects of ultrasound waves — it uses the conversion of acoustic energy into heat. The system consists of two main parts: a high-frequency electric current generator and an application head of its own ultrasound source formed by a piezoelectric transducer. The heating of tissues depends on their physical properties and their blood supply. The highest heating is at the interfaces between tissues, which are strongly different from each other in acoustic impedances.
- Microwave therapy a device called a magnetron that emits strong magnetic waves; microwaves set electrically charged particles into oscillating motion, which is transformed into heat by friction. It is mainly used in ophthalmology and otorhinolaryngology.
Induced currents', eddy currents (Foucault currents), arise in tissues exposed to high-frequency current therapy. Electrons in the tissue begin to move in circles and the tissue is heated.
Magnetic properties of muscle tissue[edit | edit source]
The electromagnetic field is created during the 'movement of electric charges.
The magnitude of the magnetic field of a current-carrying conductor can be calculated as follows:
μ...permeability of the medium,
I...the current passing through a conductor (the conductor in this case is muscle tissue),
d...the distance of the point from the conductor.
Of course, this formula cannot be directly applied to muscle tissue, which is not an ideal direct conductor.
Therefore, it also arises in muscles - as a result of local ion currents, during depolarization and repolarization of muscle cells. These magnetic fields are very weak and difficult to detect.
Using the magnetic properties of muscles[edit | edit source]
Diagnostic usage[edit | edit source]
The size of the electric currents inside the tissue is important for the formation of a magnetic field. If a larger than usual magnetic field is detected, it can be assumed that even the electric current that caused it is not completely physiological. So it can signal a health problem.
Magnetic signals can be sensed non-contact, with a superconducting quantum magnetometer (SQUID). But it is very difficult to work with because it operates at a temperature of 4.2 K, so it has to be cooled with liquid helium. In addition, it is very sensitive, so it also captures interfering fields.
Magnetic Resonance (MR) is among the most complex investigative methods. The principle of this method is computer monitoring of changes in the behavior of various cells in the human body under the influence of a strong magnetic field. The construction of imaging systems is based on the phenomenon of nuclear magnetic resonance and nuclear magnetic resonance spectroscopy. MR has a privileged position in the diagnosis of degenerative diseases of the CNS, vascular events, congenital defects, and especially tumors of the brain and spinal cord. Since a very strong signal can be obtained from adipose tissue with MR, it is possible to use it to diagnose the bone marrow and mediastinum. Unlike CT, it enables easy differentiation of vessels from solid tissues (lymph nodes, tumors). Furthermore, MR can be used in the non-invasive diagnosis of diseases of the locomotor system, e.g. cartilage lesions. The use of MR in cardiovascular diseases using MR angiography is also beneficial. An indisputable advantage is that during this examination the patient is not exposed to ionizing radiation. However, patients with pacemakers or objects made of magnetic materials cannot be examined. The disadvantage is the high price of the examination - in the order of several thousand crowns.
Medicinal use[edit | edit source]
Magnetotherapy uses pulsed magnetic fields to treat certain diseases. Applicators are wires wound into coils of various shapes - solenoid, sheet, disk, tunnel. The magnetic induction of the sources varies between 2-70 mT, pulse widths from a few ms to tens of ms.
Magnetic field effect:
- Affects electron interactions at the atomic and subatomic level (magnetic resonance, influencing electron spin).
- Magnetomechanical effect (changes in orientation of some macromolecules - DNA, RNA, bipolar water molecules, changes in BM permeability for various ions,...).
- Magnetoelectric effect (creation of induced potentials on anatomical structures and biological units).
Pulse magnetotherapy increases the proliferative activity of stem blood cells in the bone marrow, the phagocytic activity of macrophages, the activation of the immune system, the consumption of oxygen in the exposed tissue, the release of spasms of smooth and skeletal muscle cells. It is used to relieve pain, support the treatment of acute and chronic inflammations, degenerative joint diseases, Bechterev's disease, neuritis, skeletal muscle spasms, some skin diseases, post-traumatic conditions with swelling and blood sprains. It has a healing effect on leg ulcers, burns, bedsores, hard-to-heal bone fractures. It cannot be used in the area of the heart, over pacemakers, during pregnancy, in malignant tumors, tuberculosis, in bleeding conditions and in severe infectious diseases with high temperatures.
Electrical and magnetic properties of the heart[edit | edit source]
Characteristics of the myocardium[edit | edit source]
 Myocardial cells are divided into:
Myocardial cells are divided into:
'Cardiac Conduction System (PSS) - "pacemaker cells" (pacemaker = rhythm setter), generate and relatively quickly distribute impulses in a certain order throughout the myocardium (they signal the cells of the working myocardium to contract ). It coordinates the contractions of individual heart sections,
working myocardium - "nonpacemaker cells", perform (ensure) their own contraction of the heart muscle.
The heart is capable of generating repetitive stimuli for its own contraction - the autonomic nervous system only regulates the frequency of contractions.
Generation of electrical biosignals:
- at the cellular level: membrane potential, osmotic phenomena, action potential (see below),
- at the tissue level: summation potential,
- at the organs' level: summation potential, e.g. ECG (see below).
Quiet potential[edit | edit source]
In the resting state, the cell is polarized. This means that intracellularly (= inside the cell, on the inside of the membrane) it is negatively charged - the concentration of anions is greater than that of cations. Extracellular (= outside the cell, on the outside of the membrane) , on the other hand, the charge is positive - the concentration of cations is greater than the concentration of anions.
Intracellular:
- The concentration of K+ cations is 150 mmol.
- The concentration of Na+ cations is 20 mmol.
- The concentration of Ca2+ cations is 0.0001 mmol.
- In addition, there are negatively charged proteins (c = 150 mmol) that cannot pass through the cell membrane.
Extracellular:
- The concentration of K+ cations is 5 mmol.
- The concentration of Na+ cations is 150 mmol.
- The concentration of Ca2+ cations is 2.5 mmol.
The total resting potential of the cell varies between -70 and -90 mV. This potential is calculated according to Nernst's equation from the concentrations of individual ions and their equilibrium potentials' (which are an important factor in the behavior of ions towards the membrane, see below) - for K+ it is -96 mV, for Na+ +52 mV.
The cell membrane (BM) is differently permeable to different ions - different ion channels (voltage-gated channels - channels for different ions open at different voltages). The most permeable membrane is for K+ ions (about 90%) - these ions "open" channels for other ions. The most important ions that influence cell polarization: K+, Na< sup>+, Ca2+, Cl-, proteins with a negative electrical charge. So that the ions do not freely diffuse through the membrane "as they please" - according to their equilibrium potentials - the cell maintains this resting state with a system of ion pumps that work thanks to the supply of energy in the form of ATP.
Individual ions:
Potassium
Thanks to its layout outside vs. inside the cell tends to release away from the cell - 'chemical gradient'. Since the cell membrane is almost 100% permeable to potassium cations, this is theoretically the case. As the concentration of potassium cations decreases intracellularly, the negative charge on the inner side of the membrane rises, causing K+ ions to be drawn back into the cell - the electrical gradient'. When the chemical and electrical gradient is in balance (no potassium cations penetrate into or out of the cell), we speak of the 'equilibrium potential', which for K+ ions is - 96 mV (according to the Nernst equation). "Potassium cations would therefore diffuse into the cell without supplying energy in the form of ATP and thus reduce the polarization of the cell (the charge inside the cell would become more "positive").
Sodium
The extracellular concentration of sodium cations is greater than intracellular, so they tend (according to the concentration gradient, chemical gradient) to diffuse into the cell. The equilibrium potential for Na+ ions (calculated by the same principle as for potassium cations) is +52 mV. The permeability of the resting cell membrane for Na+ is small, so that even if these cations are pulled in with a relatively large force (-142 mV = difference of resting potential BM -90 mV and equilibrium potential +52 mV), sodium into the cell it only diffuses very slowly.
Calcium
Due to the distribution of calcium on different sides of the BM, it is pulled towards the cell with a large force (-224 mV), but this is only manifested during the 'action potential (AP) The equilibrium potential also corresponds to the force that the cell must exert to keep the ions in the positions desired for the resting potential of the cell membrane.
Methods of maintaining the cell's resting potential:
Sodium-Potassium Pump
Located in the BM, it works on the principle of supplying energy in the form of ATP. Principle: 3Na+ from the cell vs. 2K+ into the cell (ie +++ out/++ in). It thus contributes to the electronegative charge on the inner side of the BM. After each AP cycle, the BM returns to the resting state.
Ca ATP pump' - "sodium-calcium exchange system"
It pumps Ca2+ out of the cell against a chemical gradient, working without the need to supply energy. 3Na+ from the cell vs. 1Ca2+ into the cell (ie +++ out/++ in). Note: during depolarization, due to the massive diffusion of sodium from the cell, this system reverses - Ca2+ flows into the cell (if the Na-K pump is inhibited, this system ensures cardiomyocyte contraction).
Principle of function at the cellular level[edit | edit source]
The whole process is based on the behavior of the BM at different voltages, i.e. on the opening and closing of voltage-controlled ion channels'. Another important factor is the different properties of the pacemaker cells and the cells of the working myocardium'.
- Pacemaker cells - formation of AP:
- Phase 4 - spontaneous depolarization: -60 mV: at the end of repolarization - opening of slow Na channels, the so-called "funny channels", located in the cells of the SA node, the AV node and in Purkinje fibers.
- Phase 0: -50 mV: opening of temporary T-type Ca channels (transient = temporary).
- Phase 3: -40 mV: opening of L-type Ca channels (long lasting).
- Phase 4: +10 mV: Ca channels close and K channels open. K+ flows out of the cell in the direction of the chemical gradient, reducing the positive intracellular charge.
- Working myocardial cells - AP transmission:
- Phase 4: -90 mV: resting membrane potential = diastole'. K+ inside the cell, Na+ and Ca2+ outside – maintain ion pumps.
- Arrival of excitation from a neighboring cell.
- Phase 0: opening of fast Na channels, influx of Na+ into the cell, depolarization (membrane potential = +30 mV).
- Phase 1: +30 mV: start of repolarization: opening of slow K channels.
- potential drop from +30 mV slightly to zero: opening of slow L-type Ca channels. Ca enters the cell: slowing of repolarization.
- Phase 2: increased level of Ca2+ intracellularly causes release of Ca2+ from the sarcoplasmic reticulum (in the cardiomyocyte). Ca2+ cations bind to troponin C and thus induce contraction = systole '.
- Phase 3: closure of L-type (slow) Ca channels, excretion of K+ from the cell in the direction of the electrochemical gradient.
- Repeating the entire cycle.
The basis for understanding cardiac muscle contraction is knowledge of the principle of skeletal muscle contraction:
Principle of skeletal muscle contraction see above
Principle of function at the tissue level[edit | edit source]
Pacemaker cells are able to generate impulses (action potentials) themselves, the cells of the working myocardium only react to them (just like normal skeletal muscle).
→ SA node: is located below the epicardium in the wall of the right atrium. It is the primary pacemaker (= rhythm setter), the excitement is created here. The frequency is 60-100/min. Spontaneous diastolic depolarization occurs faster here than elsewhere in the heart.
→ internodal (= between two nodes) atrial connections:receive an impulse (=wave of depolarization) from one node to another (and to the working myocardium of the atria) using preferential routes. Here again, depolarization occurs faster than it would occur in the working myocardium.
- Bachmann pathway (from right ventricle to left ventricle)
- Wenckebach volume
- James's volume
- Thorel's Volume
→ AV node: is in the area below the endocardium in the wall of the right atrium. It conducts the impulse very slowly (desirable) - atrioventricular conduction delay (AV, atrioventricular). The contraction (depolarization) of the atria must be completed first, and only then can the contraction (depolarization) of the ventricles begin. If the SA node fails, the AV node takes over its role → secondary pacemaker (but such heart rate is then slower). The frequency is 60-90/min.
→ Bundle of His: ensures the only path of excitation (depolarization) from the atria to the ventricles.
→ Towar's arms:are divided into right and left arms. The right leads the excitation (depolarization) to the right ventricle, the left arm to the left ventricle.
→ Purkinje fibers: branch from Towar brachii. It transmits excitement to the working myocardium of the ventricles, which leads to their contraction. In extreme conditions, they can take over the function of a pacemaker, the frequency is then very slow: 30-40/min.
Principle of function at the organ level[edit | edit source]
Electric activity of the heart
The heart is a hollow muscular organ adapted to long-term work. It is made up of striated muscle, but the duration of the action potential is up to a hundred times longer than that of skeletal muscle. Fast depolarization is caused by the influx of sodium ions (fast channels) into the myocardial fiber, its long duration is caused by calcium ions. The long duration of the AP causes depolarization of cardiac muscle fibers along their entire length.
The phases of cardiac contraction can be summarized as follows: polarization (the surface of the fiber is positively charged), depolarization (the surface gradually changes polarity), transpolarization (the surface is negatively charged), repolarization (return to the original polarity).
During depolarization and repolarization, the fiber behaves like an electric dipole and becomes a generator of local currents (ion flows). The heart can be considered a complex dipole, its direction corresponds to the peak of the oscillation R on the electrographic curve, it is marked as the axis (vector) of the heart. The muscles of the atria and ventricles represent two separate sections.
The irritation of the heart spreads from the sinoatrial node (there is depolarization of the ventricles and repolarization of the atria) to the atrioventricular node at the interface of the atria and ventricles, where the conduction system of the ventricles begins: the bundle of His and the Purkinje fibers. Finally, repolarization of the ventricles occurs. In this way, the distribution of irritation along the ventricular muscle and the proper function of the heart chambers is ensured.
Magnetic activity of the heart
See magnetic properties of muscles.
Using the electrical properties of the heart[edit | edit source]
Diagnostic methods[edit | edit source]
Electrocardiography (ECG) is one of the basic examination methods in cardiology. It provides us with a graphic record of the electrical activity of the heart as an accompanying manifestation of the activity of the heart cells. It is related to their excitability and transference of irritation. By monitoring the changes, information can be obtained about disorders of the origin and spread of irritation in the heart muscle. It is closely related to mechanical activity.
The electrocardiographic curve can be explained on the basis of knowledge of the electrical properties of the heart, i.e. the generation of electrical potentials and the activation process of individual sections of the heart:
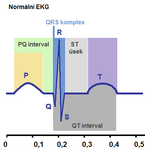
- P = depolarization of the atria (their starting contraction) = atrial systole
- QRS = ventricular depolarization = ventricular systole'
- atrial repolarization cannot be read on the ECG curve, its signal is overshadowed by the ventricular depolarization signal
- T = ventricular repolarization = ventricular diastole'
It is assumed that the potentials sensed on the surface of the body arise at the interface between the polarized and non-polarized part of the myocardium, that is, the ECG curve describes the progress of this wave. An unexcited or completely exasperated myocardium does not cause any potential change visible on the ECG.
The heart is surrounded by a diverse spatial conduit of body tissues and fluids. Therefore, there are complex changes in the manifestations of the heart's own electrical activity on the way from the heart muscle cells to the place where we most often observe it - on the surface of the body. It is also affected by the change in the position of the heart during breathing, the filling of the heart chambers with blood, cardiac hypertrophy and a number of other factors.
Treatment methods[edit | edit source]
Cardiac stimulation
It is used in cases where heart automation disorders occur - it helps maintain the heart rhythm in physiological values. There is external and internal stimulation. External stimulation is used only for short-term procedures, internal stimulation prevails, when the electrode is inserted into the heart via a venous catheter using an implanted pacemaker under the skin on the chest.
The pacemaker automatically engages when it recognizes that the heart rate has slowed down below the set value.

Defibrillation
Defibrillation is the most effective and often the only life-saving therapy ventricular fibrillation. Ventricular fibrillation is an acute, life-threatening condition. It causes uncoordinated tremors of the heart muscle, the heart stops working as a pump and blood circulation slows down to a stop.
The electrical discharge simultaneously depolarizes all the cells of the myocardium and thereby induces the conditions for the activation of the physiological centers of the generation and propagation of the excitation.
The device used to defibrillate the ventricles is called a defibrillator. The energy chosen to terminate ventricular defibrillation must be suprathreshold. It ranges from 200 J to 360 J.
During the shock, for safety reasons, no one must be in conductive contact with the defibrillated patient, and the oxygen source must also be removed.
Cardioversion
Electrical cardioversion is a planned electrical shock therapy for paroxysmal (convulsive) ventricular tachycardia or for atrial fibrillation or flutter (oscillation) as a supplement to pharmacotherapy. Requires less energy than defibrillation: 50-100 J.
ICD (Implantable Defibrillator-Cardioverter)
ICD is a device combining both previous procedures. Its electrodes are introduced transvenously, similar to the implantation of a pacemaker. It can distinguish sudden ventricular fibrillation from ventricular tachycardia and has the function of a stimulator ensuring the basic heart rhythm.
Using the magnetic properties of the heart[edit | edit source]
Diagnostic methods[edit | edit source]
Magnetocariography (MKG) is a technique used to measure the magnetic fields produced by the electrical activity of the heart muscle. Extremely sensitive devices are used, such as a ``SQUID (superconducting quantum magnetometer. If devices with multiple outputs are used for measurement, we obtain a map of the magnetic field of the entire chest. From such a map, we can use mathematical procedures that must take into considering also the conductivity of the chest structure, to locate the source of electrical heart activity. Magnetocardiography can be used to investigate the causes of an abnormal heart rhythm or arrhythmia.
Fetal magnetocardiography (fMKG), as the name suggests, is used to examine heart activity in the fetal stage of development.
Treatment methods[edit | edit source]
Magnetotherapy is not recommended to be applied in areas of the heart, however theoretically it can affect diseases related to heart activity, such as hypotension, hypertension or even ischemic heart disease.
Links[edit | edit source]
Related Articles[edit | edit source]
References[edit | edit source]
- BLAHÚT, Peter. Action potential and heart [online]. [cit. 2015-03-29]. <https://www.techmed.sk/akcny-potential-a-srdce/>.
- Wikiscript. Connection of excitation and contraction [online]. [cit. 2015-03-29]. <https://www.wikiskripta.eu/w/Spojen%C3%AD_excitace_a_kontrakce>.
- Wikiscripta. Neuromuscular plate [online]. [cit. 2015-03-29]. <https://www.wikiskripta.eu/w/Nervosvalov%C3%A1_plot%C3%A9nka>.
- Wikiscripta. Electrostimulation methods [online]. [cit. 2015-03-29]. <https://www.wikiskripta.eu/w/Elektrostimula%C4%8Dn%C3%AD_metody>.
- Wikipedia. Magnetocardiography [online]. [cit. 2015-03-29]. <https://en.wikipedia.org/wiki/Magnetocardiography>.
- DIMAP s.r.o.. Knowledge, physiological and pathophysiological responses to the action of magnetic fields, magnetotherapy [online]. [cit. 2015-03-29]. <https://www.dimap.cz/index.php?page=interier&id=002&idsub=0005>.
- PROKOP,. Biophysics of tissues and organs [online]. The last revision 2009-10-07, [cit. 2015-03-29]. <http://media0.mypage.cz/files/media0:4ad5bf70aabba.doc.upl/Biofyzika%20tkani%20a%20organu.doc>.
- KÓTÁLEK, Jacob. Generator of pathological ECG curves for the needs of simulation models. Prague : Czech Technical University, Faculty of Electrical Engineering, Department of Cybernetics, 2010,
- NAVRÁTIL, Leoš – ROSINA, Joseph, et al. Medical Biophysics. 1 (reprint 2013) edition. Prague : Grada Publishing, 2005. 524 pp. ISBN 978-80-247-1152-2.
- VAJNER, Ludek, et al. Medical histology I : General Cytology. 1. edition. 2010. ISBN 978-80-246-1860-9.