Congenital defects of metabolism with acute symptomatology
Congenital defects of metabolism are a very extensive and heterogeneous group of diseases. They are caused by faulty function of one or more enzymes or changes in the composition or quantity of structural or transport proteins. They are usually inherited autosomal recessive or gonosomal recessive; mitochondrial diseases have a maternal type of inheritance. Some of them manifest themselves already in the newborn period. Some of these disorders are targeted by newborn laboratory screening.[1]
The clinical manifestation of congenital metabolic defects can involve virtually any system. The most common are neurological and gastrointestinal symptoms. Manifestations can be acute or chronic. Acute symptoms include: vomiting with dehydration to shock, lethargy and coma, rhabdomyolysis, hypoglycemia during illness, stress or prolonged starvation. The chronic symptoms include: signs of metabolic disease with growth failure/delay, hepatomegaly, cardiomyopathy, spastic diplegia, delay in psychomotor development or regression in development.[2]
The first symptoms of congenital metabolic defects can appear at any age from the prenatal period to the elderly, and they can manifest differently at different ages. The onset and severity of difficulties are affected by a number of factors, such as a change in diet, starvation, dehydration, ongoing illness, medication, exertion, childbirth, trauma, surgery.[2]
For some defects, the typical age of manifestation' is:
- non-ketotic hyperglycinemia, urea cycle disorders, organic acidemia (of branched chains) present as a life-threatening illness (lethargy, anorexia, vomiting, shock) between the 12th and 72nd hours of life,
- maple syrup disease usually appears later in the first week of life.[2]
- neonatal hemochromatosis is manifested by acute hepatic failure in the first week of life,
- galactosemia is manifested by acute liver failure in the first or second week of life,
- tyrosinemia manifests as acute liver failure anytime after the first week of life,
- alpha1-antitrypsin deficiency, Niemann-Pick disease, defects in bile acid synthesis are manifested by acute liver failure after the third week of life,
- mitochondrial disease can occur at any time.[2]
Common laboratory findings include:
- metabolic acidosis/alkalosis, hyperlactic acidemia, hyperammonemia, elevation of liver enzymes, hypoglycemia, ketosis.
Therapy before diagnosis:
- treatment of cardiopulmonary failure, total parenteral nutrition with protein restriction (0.5-0.8 g/kg/day) and without lipids, the energy source is glucose (except for pyruvate dehydrogenase complex defects).
Therapy after diagnosis:
- pharmacotherapy to induce an alternative metabolic pathway, in case of accumulation of toxic metabolites due to enzyme block, eliminative treatment (hemodialysis or hemodiafiltration), dietary measures.[1]
Congenital defects of newborns' metabolism[edit | edit source]
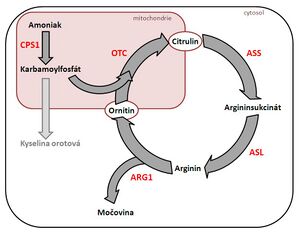
Urea cycle disorders[edit | edit source]
- the consequence of defects is hyperammonemia → serious neurological consequences and multi-organ failure;
- the most severe forms begin in the first days (refusal of food, apathy, hypotonia, convulsions → vomiting, progression of impaired consciousness, hypothermia, bleeding manifestations, circulatory failure) and have a lethal course;
- laboratory findings: hyperammonemia;
- autosomal recessive or gonosomal recessive inheritance;
Leucinosis (maple syrup disease)[edit | edit source]
- impaired activity of dehydrogenases for branched-chain 2-oxoacids, which are formed from the branched-chain amino acids valine, leucine and isoleucine → neurotoxic acids (2-oxoacids and 2-OH-acids) accumulate → disorders of food intake and vomiting, disorders muscle tone, impaired consciousness → brain edema, circulatory and respiratory failure;
- laboratory findings: ketoacidosis;
- autosomal recessive inheritance;
Nonketotic hyperglycinemia[edit | edit source]
- dysfunction of the mitochondrial enzyme complex that splits glycine (neurotransmitter – excitatory in the cerebral cortex and inhibitory in the medulla oblongata and spinal cord; importance in the metabolism of hemoglobin, purines and creatinine) → accumulation of glycine, especially in the CNS → convulsions (pharmacologically difficult to influence);
- laboratory finding: elevation of glycine in cerebrospinal fluid (compared to plasma);
- autosomal recessive inheritance;
- unfavorable prognosis, only symptomatic treatment;
Organic aciduria[edit | edit source]
- lead to the accumulation of carboxylic acids without a free amino group, which are excreted in the urine;
- laboratory findings: hyperlactic acidemia, ketoacidosis, hyperammonemia; pancytopenia; hemocoagulation disorders;
Disorders of carbohydrate metabolism[edit | edit source]
Galactosemia[edit | edit source]
- galactose-1-phosphaturidyl transferase disorder → accumulation of galactose-1-phosphate → 'nephrotoxic, hepatotoxic and neurotoxic galacticol;
- manifestations after starting milk nutrition → loss of appetite, vomiting, weight loss, hepatomegaly, lethargy → liver and kidney failure, edema, ascites, brain edema;
- laboratory findings: elevation of aminotransferases, unconjugated hyperbilirubinemia, hypoglycemia, hemocoagulation disorder, anemia, etc.
- diagnostics: galactitol in urine and increased values of galactose and galactose-1-phosphate in erythrocytes → enzymatic and/or molecular diagnostics;
- autosomal recessive inheritance;
Persistent hyperinsulinemic hypoglycemia[edit | edit source]
- insulin hypersecretion → severe hypoglycemia already in the first days of life;
- treatment: glucose, glucagon, diazoxide, octreotide, possibly subtotal pancreatectomy;[1]
Disorders of mitochondrial energy metabolism[edit | edit source]
- mitochondrial diseases are hereditary metabolic diseases caused by mutations either in the nucleus in the genes for mitochondrial enzymes or in mitochondrial DNA (non-Mendelian maternal inheritance), which have different clinical manifestations;
- the citrate cycle, oxidative phosphorylation, β-oxidation of fatty acids, part of the urea cycle take place in the mitochondria;
- disorders of energy metabolism:
- clinical picture: psychomotor retardation and developmental regression, myoclonic epilepsy, central hypotonic syndrome or spastic quadriparesis;
- laboratory finding: "increased lactate and alanine level" in blood, urine and cerebrospinal fluid; in myopathic manifestations, the level of creatine kinase is increased; aminotransferase elevation in hepatopathy.[1]
Disorders of beta-oxidation of fatty acids[edit | edit source]
- typical finding: hypoketotic hypoglycemia during starvation.
Odkazy[edit | edit source]
Related Articles[edit | edit source]
External links[edit | edit source]
References[edit | edit source]
- ↑ a b c d JANOTA, Jan – STRAŇÁK, Zbyněk. Neonatologie. 1. edition. Praha : Mladá fronta, 2013. pp. 259-269. ISBN 978-80-204-2994-0.
- ↑ a b c d SUTTON, V R. www.uptodate.com [online]. UpToDate, ©2020. [cit. 2023-02-15]. <https://www.uptodate.com/contents/inborn-errors-of-metabolism-epidemiology-pathogenesis-and-clinical-features>.
- ↑ GOMELLA, TL – A KOLEKTIV,. Neonatology : Management, Procedures, On-Call Problems, Diseases, and Drugs. 7. edition. Lange, 2013. pp. 686-709. ISBN 978-0-07-176801-6.