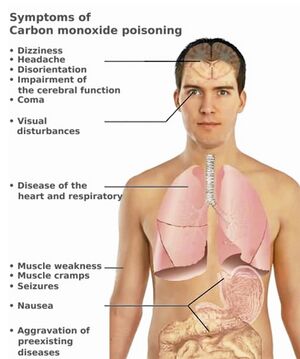
Carbon monoxide poisoning
Carbon monoxide is a non-irritating, odorless gas. It is lighter than air but mixes freely with air. Its presence in the possessions is thus influenced rather by air currents. It is formed by incomplete oxidation of carbonaceous substances. Poisoning is very often overlooked.
More detailed information can be found on the Oxides of carbon page .
Professional exposure[edit | edit source]
- Risk workplaces - boiler rooms, lignite mines, where coal smolders (mine gases contain up to 50% CO, water gas 38%, exhaust gases 11%),
- Non-professionally - from natural gas (bathrooms - karmas).
Etiopathogenesis[edit | edit source]
Binding to Hb is about 210× higher than to oxygen . It forms carboxyHb and thereby occupies binding sites for oxygen. The consequence is tissue anoxia. As COHb increases, the oxygen dissociation curve shifts to the left. COHb has a bright red color, which is most evident in venous blood. The formation of CO 2 decreases , causing hypocapnia. CO also binds to cytochrome P450 and myoglobin , resulting in a decrease ( myocardial contractility ). Apparently, it also has a specific cytotoxic effect. In addition to the concentration in the air, the physical load also matters - firefighters breathe the most (higher minute volume). The first organs to be damaged are the brain and myocardium due to the highest demands for oxygen (there is a physiological decrease in blood flow in other organs).
The binding of COHb is reversible - it makes sense to take the affected person to fresh air. During a stay in the fresh air, COHb decreases by half in 4 hours (with oxygen therapy in an hour).
In non-smokers, the usual presence of COHb is about 1%, in smokers 5% or more.
Clinical picture[edit | edit source]
Acute[edit | edit source]
They are not specific, to verify the diagnosis it is necessary to determine the level of COHb. In addition to the laboratory, a pulse co-oxymeter can be used - a device capable of distinguishing the wavelengths of COHb and HbO2. Conventional oximeters do not differentiate between these hemoglobins and report a falsely acceptable saturation value in a severely hypoxic patient.
- 10% COHb – slight concentration disorder;
- 20% COHb – mild headache, dizziness ;
- 30% COHb – headache , nausea , vomiting , exertional dyspnea ;
- 40-50% COHb – confusion, persistent headache, even coma and convulsions ;
- above 60% COHb - deep coma, death.
Chronic[edit | edit source]
Because the binding is reversible, chronic poisoning is usually denied. After acute poisoning with unconsciousness, the consequences persist.
- Pseudoneurasthenic syndrome , extrapyramidal symptoms, possibly organic psychosyndrome ;
- neurological symptoms – parkinsonism with mutism , agnosia , visual disturbances;
- personality changes, increased irritability, verbal aggression;
- we often find necrosis in the globus pallidus , as well as in other BGs , the hippocampus and white matter.
Investigation methods[edit | edit source]
Blood sampling for COHb determination must be done as soon as possible. Measurement of saturation (pulse oximetry or sampling) - falsely normal results (bright red COHb color). In the acute stage – we find MAC , increased glycemia . Changes in the CNS – CT , MRI . PET can detect ischemic foci in an acute state (however, it is difficult to estimate the prognosis from this).
Therapy[edit | edit source]
Remove immediately to fresh air. Causal treatment is oxygen therapy , in severe poisoning – hyperbaric oxygen therapy .
Links[edit | edit source]
External links[edit | edit source]
- Carbon monoxide poisoning — interactive algorithm + test
- Website of the Ministry of Health of the Czech Republic
Source[edit | edit source]
- BENEŠ, Jiří. Studying Materials [online]. [cit. 24.02.2010]. <http://jirben.wz.cz>.
References[edit | edit source]
- PELCLOVÁ, Daniela. Occupational diseases and intoxication. 2. edition. Karolinum, 2006. 207 pp. ISBN 80-246-1183-X.
Categorie:Occupational medicine Categorie:Pathobiochemistry Categorie:Chemistry Kategorie:Toxikologie