Advance glycation products
Advanced glycation endproducts ( AGEs ) are a heterogeneous group of substances , including pentosidine, GOLD (glyoxal-lysine dimmer), MOLD (methylglyoxal-lysine dimmer) .
They are characterized by yellow-brown pigmentation and fluorescence . They are able to modify biological structures . They react with specific receptors, eg RAGE . They are important in the pathogenesis of late complications of diabetes mellitus and chronic diseases such as chronic renal failure , atherosclerosis , neurodegenerative diseases and others.
Proteins change their physical and chemical properties during their glycation and subsequent changes . They include, for example, changes in solubility, charge and isoelectric point, crosslinking , increased resistance to thermal denaturation and stability to lowering pH .
AGEs arise as advanced products of non-enzymatic protein glycation. This process is closely related to oxidative stress and carbonyl stress.
Non- enzymatic glycation[edit | edit source]
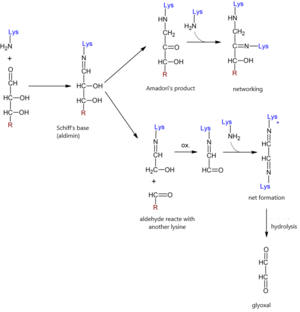
In non-enzymatic glycation of proteins, the free amino groups of proteins (especially the lysine and arginine side groups, to a lesser extent the histidine side groups) react with the carbonyl groups of reducing sugars without the catalytic action of enzymes . This reaction was first described by Louis Maillard, who observed the browning of proteins when heated with sugars.
Maillard's reaction[edit | edit source]
- Initiation
The reaction is initiated by non-enzymatic condensation of the aldehyde group of the reducing sugar and the amine group to form aldimine ( Schiff base ). The reaction proceeds rapidly and equilibrium occurs within a few hours. This reaction is easily reversible.
- Promotion
Schiff's base undergoes chemical rearrangement within a few days to form more stable structures, which are referred to as Amadori products . These structures have the character of ketoamine . Amadori's products are to some extent reversible, their balance is significantly shifted in the direction of their creation. Steady state occurs within 20-30 days.
Other reactions can form carbonyl compounds such as glyoxal, methylglyoxal, deoxyglucosone.
Deoxyglucosone is formed at higher pH, when Amadori products enolize in the position between the second and third carbon and thus eliminate the amine from the first carbon. These compounds are highly reactive and are intrinsic promoters of non-enzymatic glycation, which cause irreversible molecular changes in proteins .
- Formation of advanced glycation products
In the last phase, Amadori products or other compounds react with the free amino groups of long-lived proteins ( collagen , elastin , myelin ). Advanced glycation products - AGE-compounds - are formed . Their formation is practically irreversible and AGEs permanently damage the tissues in which they are stored
Oxidative Stress[edit | edit source]
An imbalance between the formation of reactive forms and antioxidants that remove them from the body. The resulting equilibrium is shifted in favor of reactive forms.
Reactive forms include hydroxyl radical, superoxide and compounds formed from Cl, NO 2 - , H 2 O 2 , Fe, Cu, as well as herbicides, pesticides and others. These substances damage biological structures - lipids (lipoperoxidation), proteins (protein cross-linking), sugars (glycosylation) and NK ( DNA mutations ).
Antioxidants ( vit.C , vit.E , selenium , Beta-carotene ) can be removed from the body .
More detailed information can be found on the page Basic reactive forms of oxygen and nitrogen .
You can find more detailed information on the page Antioxidant protection of the human body .
Carbonyl stress[edit | edit source]
An increase in reactive carbonyl compounds leads to gradual organ damage . It can be caused by their increased production or their reduced degradation (elimination error - aldehyde dehydrogenase) and subsequent excretion.
Carbonyl compounds are very closely related to oxidative stress, hyperlipidemia and hyperglycemia. Carbonyl compounds include glyoxal , glycoaldehyde , hydroxynonenal , methylglyoxal , 2-deoxyglucosone . These compounds can be formed from carbohydrates, amino acids and fats.
See the Carbonyl Stress page for more information .
RAGE receptor[edit | edit source]
RAGE is a transmembrane protein that serves as a receptor for advanced glycation products. They belong to the immunoglobulin superfamily. It most commonly occurs on endothelial cells (areas typically affected by atherosclerosis), macrophages , and microglia in brain tissue.
AGEs-RAGE interactions[edit | edit source]
Interactions between AGEs-RAGE cause intracellular signaling. It also leads to oxidative stress and activation of MAP kinases. These two mechanisms lead to the activation of transcription factors such as NF-κB (nuclear factor kappa B). NF-κB affects the expression of genes important for immunity, inflammatory response, cell growth, cell death and embryonic development.
The action of the RAGE receptor in the body[edit | edit source]
Activation of NF-κB stimulates the production of cytokines (IL-1, TN-α, interferon γ) and growth factors (IGF-1, PDGF). This results in the expression of adhesive molecules, increased cell proliferation, increased vascular permeability. It also stimulates macrophage migration, endothelin production. The synthesis of collagen IV, proteoglycans and fibronectin increases . At the site of inflammation , it stimulates the formation of carboxymethyllysine (CML) in phagocytes.
Negative effects of AGEs in the body[edit | edit source]
Complications of Diabetes mellitus[edit | edit source]
Chronic changes in diabetes are the result of hyperglycemia, which leads to increased protein glycation and subsequent oxidative and carbonyl stress. Carbonyl and oxidative stress alone leads to the formation of AGEs and ALEs.
However, this mechanism is not the only one that leads to organ damage in diabetes. For example , hyperglycemia alone increases the amount of AGEs and ALEs (non-enzymatic glycation) - a disorder of lipid metabolism. It should be noted that the development of complications in diabetes is not caused by a single mechanism. It is a complex and to some extent cascading process that is highly interconnected.
- Metabolic changes
Non-enzymatic glycation - Maillard reaction.
Intracellular hyperglycemia occurs in tissues where insulin is not required (eye lens, nerve tissue, kidneys). Glucose is metabolized to sorbitol and fructose , which causes hyperosmolarity of the cells and the resulting osmotic damage to the cell . Sorbitol also damages ion pumps , leading to neuropathies and aneurysms in the retina .
- Macrovascular complications
Accelerated development of atherosclerosis, which leads to coronary heart disease.
- Microvascular complication
Nephropathy leads to kidney failure. Deposits are stored in the basement membrane, which leads to its thickening and charge change. Furthermore, growth factors are secreted, vascular permeability increases, densification and mesangial matrix increase. Vascular wall proteins are modified (crosslinking). Glycation and oxidation of LDL particles and collagen occurs, leading to endothelial damage.
- Retinopathy
- Non-proliferative form - microaneurysms, minor bleeding, exudates , edema .
- Preproliferative form - avascular sections, area hemorrhages.
- Proliferative form - formation of new blood vessels, fibrosis, vitreous hemorrhage.
Cardiovascular complications[edit | edit source]
Modification of vascular wall proteins (crosslinking). There is increased extracellular matrix production, glycation and oxidation of LDL particles, as well as endothelial damage (collagen glycation).
Other complications include arterial damage (weakening of the vessel wall, changes in vascular permeability) and atherosclerosis.
Complications of other systems[edit | edit source]
- Nervous system - The accumulation of AGEs in pyramidal cells (neurons) is probably related to Alzheimer's disease , in general it can be said to be a neurodegenerative disease.
- Respiratory system - Chronic lung diseases.
- GIT - Liver cirrhosis .
- Joints - Rheumatoid arthritis .
Therapeutic possibilities of reducing the effect of AGEs[edit | edit source]
Prevention of negative effects of AGEs in the body can be prevented in general at 3 levels:
- Development of AGEs - Careful compensation of diabetes (diet, reduction of hyperglycemia and hyperlipidemia, reduction of oxidative stress).
- Chemical degradation of cross-linked proteins .
- AGEs-RAGE interactions - Effect on anti-RAGEAb receptor effect. Increased expression of soluble AGE receptor (sRAGE) - inhibitor of AGEs toxic effects. Its expression is increased by some agiotensin converting enzyme (ACEI) inhibitors, such as ramipril or perindopril . Some antidiabetics block RAGE signaling (pioglitazone , empagliflozin ).
There are ongoing studies on the effects of AGEs and their side effects. In this context, we can include substances such as taurine, carnosine, aspirin, pyridoxamine, aminoguanidine, alpha-lipoic acid.
Links[edit | edit source]
[edit | edit source]
- Type 2 diabetes mellitus
- Type 1 diabetes mellitus
- Diabetes mellitus
- Defective counter-regulation syndrome
Reference[edit | edit source]
- ↑ MIYATA, T, S SUGIYAMA and A SAITO, et al. Reactive carbonyl compounds related uremic toxicity ("carbonyl stress"). Kidney Int Suppl [online] . 2001, vol. 78, pp. S25-31, also available from < https://www.ncbi.nlm.nih.gov/pubmed/11168978 >. ISSN 0098-6577.
- ↑ FORBES, Josephine M, Suzanne R THORPE and Vicki THALLAS-BONKE, et al. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J Am Soc Nephrol [online] . 2005, vol 16, no. 8, pp. 2363-72, also available from < https://www.ncbi.nlm.nih.gov/pubmed/15930093 >. ISSN 1046-6673.
- ↑ DI, Bei-Bing, Hong-Wei LI and Wei-Ping LI, et al. Pioglitazone inhibits high glucose-induced expression of receptor for advanced glycation end products in coronary artery smooth muscle cells. Mol Med Rep [online] . 2015, vol 11, no. 4, pp. 2601-7, also available from < https://www.ncbi.nlm.nih.gov/pubmed/25523934 >. ISSN 1791-2997 (print), 1791-3004.
- ↑ OJIMA, A, T MATSUI and Y NISHINO, et al. Empagliflozin, an Inhibitor of Sodium-Glucose Cotransporter 2 Exerts Anti-Inflammatory and Antifibrotic Effects on Experimental Diabetic Nephropathy Partly by Suppressing AGEs-Receptor Axis. Horm Metab Res [online] . 2015, vol. Epub ahead of print, pp. Epub ahead of print, also available from < https://www.ncbi.nlm.nih.gov/pubmed/25611208 >. ISSN 0018-5043 (print), 1439-4286.
References[edit | edit source]
- Advanced glycation end-product wikipedia.org
- KALOUSOVA, Marta, et al. Pathobiochemistry in schemes. 1st edition. Prague: Grada, 2006. ISBN 80-247-1522-8 .
- TOMÁŠ OBŠIL, ZDENĚK PAVLÍČEK. GLYCATION OF PROTEINS AND PHOSPHOLIPIDS: MAILLARD'S REACTION IN VIVO [online]. [feeling. 2011-01-16]. < http://www.chemicke-listy.cz/docs/full/1997_08_558-569.pdf >.
Source[edit | edit source]
- KRTIL, Jan. Non-enzymatic glycation, insulin resistance, metabolic syndrome [online]. © 2017. [feeling. 16.12.2018]. < https://ulbld.lf1.cuni.cz/file/2889/metabolic-syndrome-2017.pdf >.