Ventilation-perfusion ratio
The ventilation-perfusion ratio is a quantity that expresses the ratio of alveolar ventilation Va and capillary flow (perfusion)Q in the lungs. This ratio can be applied to the whole lung or to a specific part. In an ideal situation, the ratio of alveolar ventilation to lung perfusion is approximately one .
Under these conditions, the transition of gases ( oxygen and carbon dioxide) between the lumen of the alveoli and the blood is most efficient.
Ventilation-perfusion imbalance[edit | edit source]
The ideal situation when,Va/Q = 1 does not always apply, not even physiologically.
Ventilation-perfusion imbalance is a condition where the ratio is Va/Q deviated from 1.
This usually happens by reducing ventilation or perfusion. This imbalance can occur locally or throughout the lungs.
Increase in ventilation-perfusion ratio[edit | edit source]
An increase in the ratio occurs, for example, when ventilation is increased, or when capillary flow is reduced or stopped. In this way, air from the surroundings is brought into the alveolus faster than CO 2 is brought in and O 2 is removed by the blood. This gradually increases pO 2 and decreases pCO 2 in the alveolus .
The partial pressure of blood gases in the blood can change in the same direction as in the alveoli, or in the opposite direction. It depends on the way in which the increase in the ventilation-perfusion ratio was achieved. If there is a reduction in flow (in case of pulmonary embolism), the pO 2 in the blood will decrease and the pCO 2 will increase due to the venous admixture. On the contrary, if there has been an increase in ventilation (in hyperventilation), the pO 2 in the blood will increase and the pCO 2 will decrease, just like in the alveoli. This condition can then lead to respiratory alkalosis .
Decreased ventilation-perfusion ratio[edit | edit source]
A decrease in the ratio occurs most often with airway obstruction, when ventilation decreases, while the flow around the obstructed alveoli remains preserved. In the alveolus, pO 2 decreases and pCO 2 increases . The flowing blood cannot be oxygenated and get rid of CO 2 , therefore pO 2 decreases in it and pCO 2 rises . In this direction, the partial pressures of both gases also change in the entire system basin, as the blood flowing around the non-ventilated alveoli mixes with the rest and thus reduces the resulting saturation (venous admixture).
The effect of gravity[edit | edit source]
Gravity affects both the blood in the pulmonary capillaries and the air in the pulmonary alveoli. Since the apex of the lung is at a different height than the base of the lung, different hydrostatic pressure acts on the blood in the capillaries of different parts of the lung. The air in the alveoli is affected by a slightly different atmospheric pressure, but this effect is negligible compared to the hydrostatic pressure of the blood.
Effect on ventilation[edit | edit source]
Gravity affects the amount of ventilation in individual parts of the lungs. At the base of the lungs, the negative pressure in the intrapleural cavity is smaller (or "less negative"). This is due to the hydrostatic pressure of the intrapleural fluid , which balances the negative pressure slightly. The alveoli are therefore less distended at the base than at the apex, and then expand more during inhalation and are more ventilated. However, the differences in ventilation are not nearly as great as the differences in perfusion.
Effect on perfusion[edit | edit source]
The capillary resistance , related to its lumen, and the perfusion pressure , i.e. the pressure difference at the arterial and venous end of the capillary (p a – p v ) , are decisive for the speed of blood flow through the capillary . Gravitational acceleration has the consequence that, when standing, the pressure is greater in the capillaries at the base of the lung than at its apex.
In the lungs, the alveoli press against the capillaries. Although the alveolar pressure (p A ) is different in different parts of the lungs (see above), we can now consider it constant for our needs. The alveoli can, with their p A , in certain cases oppress the capillaries, limit their lumen, and therefore reduce the flow, or even stop it completely. The greater pressure inside the capillaries allows this pressure to be compensated and thus maintain greater flow.
According to some authors, gravity is not the cause of differences in capillary flow, but this cause is different. The rationale is the fact that the difference in flow at the base and apex of the lung exists even in the weightless state of the orbit.
We can therefore divide the lungs into 3 zones
- Zone at the apex : pA > pa – The capillaries here are collapsed due to low blood pressure and the flow is zero.
- Middle zone : pa > pA >pv – Blood flows through the capillaries, but the capillary is partially compressed because the alveolar pressure is greater than the pressure at the venous end of the capillary.
- Base Zone: pa > pv > pA– The capillary is open, perfusion is not limited by the alveolus, because the pressure at the venous end of the capillary (ie the pressure in the entire capillary) has exceeded the alveolar pressure.
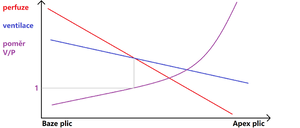
Both ventilation and perfusion decrease from the base to the apex of the lung, with perfusion decreasing faster than ventilation. At the point where the two curves intersect, the ventilation-perfusion ratio is equal to one. The differences in local perfusion are therefore very significant. At the base, perfusion is greatest, decreasing in the cranial direction. Flow in the upper zone may stop completely, but this zone represents a minimal portion of the lung . As already mentioned, the differences in perfusion are greater than in ventilation (see figure).
Ventilation-perfusion ratio control[edit | edit source]
- Areas with a reduced ratio cannot in any case replace areas with an increased ratio. In the area with a reduced ratio, the blood is insufficiently oxygenated and has a normal amount due to normal perfusion.
- On the contrary, areas with an increased ratio give more oxygenated blood, but since the transport capacity of the blood is almost exhausted under normal circumstances, the increase is slight. But there is little blood due to the low flow.
When a large amount of poorly oxygenated blood and a small amount of highly oxygenated blood mix, the result is low oxygenated blood. It is therefore necessary to constantly maintain the ventilation-perfusion ratio in all areas of the lung close to 1.
The body has a mechanism that maintains the ventilation-perfusion ratio at acceptable values. This is hypoxic pulmonary vasoconstriction . If there is a local decrease in ventilation, i.e. a decrease in V A /Q, pO 2 gradually decreases in the hypoventilated alveoli . The pulmonary arteries supplying blood to these alveoli respond by vasoconstricting, thereby reducing the perfusion of the area. Blood is then diverted and led through other arteries that have not undergone vasoconstriction to areas with normal ventilation. More ventilated alveoli are more perfused and the V A ratio/Q is favorable for efficient gas exchange in all parts of the lungs. If the ventilation of a tissue portion returns to higher values, the arterial smooth muscle relaxes, resistance decreases, and perfusion increases until a new balance between ventilation and perfusion is established. The disadvantage of this mechanism is that in general hypoventilation, e.g. as a result of airway obstruction, general pulmonary vasoconstriction occurs and the resistance of the pulmonary circulation increases significantly. The right heart then has to exert great force during its contractions to ensure sufficient flow. It is more prone to heart failure , among other things, because the blood in the coronary arteries in this situation has less and less pO 2 . The exact mechanism of hypoxic pulmonary vasoconstriction has not yet been fully elucidated.
- It is also good to be aware of the importance of hypoxic pulmonary vasoconstriction during the birth of a child, when the increase in pO 2 in the lungs results in a significant reduction in the resistance of the pulmonary blood stream . According to some authors, the prenatal development of the fetus, during which it is necessary to maintain low pulmonary flow and then to activate the lungs, is the main reason why hypoxic pulmonary vasoconstriction has been maintained in human evolution.
Right-left shunts[edit | edit source]
Anatomical right-left shunts are situations where the arrangement of the vascular system is such that part of the venous blood from the bronchial and coronary channels enters the pulmonary veins or directly into the left atrium of the heart. It is therefore deoxygenated blood that does not pass through the pulmonary capillaries and enters the systemic circulation directly, where it dilutes the normally oxygenated blood. The ratio of ventilation to perfusion is 0 in the case of shunts. In a healthy person, they represent about 2% of cardiac output .
Other shunts can occur in sick people ( pneumonia ) or people with congenital heart defects, and their effect on the overall ventilation-perfusion ratio must be taken into account.
Links[edit | edit source]
Resources[edit | edit source]
- GANONG, William F, et al. Review of Medical Physiology. 1st edition. Jinočany: H&H, 1995. 681 pp. ISBN 80-85787-36-9 .
- WARD, Jeremy and Roger LINDEN. Basics of physiology. 1st edition. Prague: Galén, 2010. 164 pp. ISBN 978-80-7262-667-0 .