S100
S100 is the name of a group of peptides with a relatively low molecular weight (9–13 kDa), which are characterized by the presence of two EF-hand domains (helix-loop-helix). S100 proteins bind calcium, some types can also bind zinc and copper. They are mainly localized intracellularly, where they participate in a number of intracellular processes, especially contractility, cell growth, differentiation, cell cycle progression, transcription, and also secretion. Some types of S100 proteins occur extracellularly, where they can interfere with some immune processes. S100 proteins are involved in a wide range of pathological conditions. Detection of S100A1 gene expression commonly referred to as detection of S100 protein in histopathology is a frequently used immunohistochemical marker.
The name S100 is derived from the property of proteins to dissolve in a saturated solution of ammonium sulfate[1] .
Molecular biology[edit | edit source]
S100 proteins are specific to vertebrates. Their structure is highly conservative. 21 peptides from the S100 family have been described, most of them localized to 1q21. Individual types are highly homologous. The expression of individual types of S100 proteins is tissue-specific, individual types form homo- and heterodimers, oligomers are also described, and S100G is the only monomeric representative.
S100 proteins are involved in a wide range of cellular events, their specific activity is determined by the specific type of protein, ion binding, and other circumstances. By binding calcium, they are involved both in calcium homeostasis of the intracellular environment and in signaling through calcium-dependent pathways. The function of the possible binding of zinc and copper is not entirely clear, but it seems that, for example, the binding of copper to S100B can have a neuroprotective effect. Extracellularly localized S100 proteins mainly interfere with immune events, for example, some S100 proteins act chemotactically, others have an angiogenic effect and others bind to RAGE (Receptor for Advanced Glycation Endproducts) receptors.
Associated diseases[edit | edit source]
Changes in the expression of S100 proteins are demonstrable in a number of diseases. These diseases can be classified into four groups:
- Cardiac diseases - Up-regulation of S100A1 in right ventricular hypertrophy and down-regulation of S100A1 in end-stage heart failure have been demonstrated. The serum level of S100A1 increases in acute myocardial infarction .
- CNS diseases – S100B in the cerebrospinal fluid is increased, for example, in brain injury, Alzheimer's disease , Downs syndrome or multiple sclerosis . S100B protein released from necrotic cells appears to enhance extracellular proapoptotic signaling. Conversely, S100B is reduced in patients with amyotrophic lateral sclerosis .
- Tumors - Changes in the expression of S100 proteins are common in tumors. Part of the changes may be due to the fact that the 1q21 region is relatively often affected by mutations in people, but part of the changes is involved in the biological behavior of the tumor. Thus, for example, the secretion of the S100A4 protein significantly increases the potential of the tumor to establish distant metastases. The S100A2 protein is located in the cell nucleus, it was originally characterized as a tumor suppressor gene, but since it has been demonstrated, for example, in the nuclei of a number of tumors, its role seems to be much more complex.
- Inflammatory diseases – S100A8 and S100A9 act chemotactically, S100A12 stimulates macrophages by binding to the RAGE receptor. Their higher level has been proven, for example, in rheumatoid arthritis or chronic bronchitis . Up-regulation of S100A8, S100A9 and S100A12 was demonstrated in psoriatic lesions.
Immunochemistry[edit | edit source]
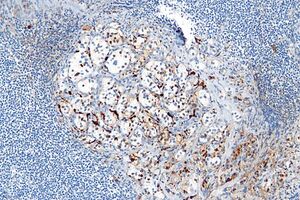
Proteins from the S100 family have a wide homology, it is technically possible to prepare antibodies that will bind to most representatives of the S100 family. Originally isolated from brain tissue, S100 is traditionally considered a marker of neuronal origin. However, it is also expressed in a number of other tissues. Especially when monoclonal antibodies are used, false negativity can occur with some types.
Normally, S100 is detectable in the following cell types:
- neuroglia and Schwann cells
- Vater-Pacini corpuscle
- melanocytes
- chondrocytes
- adipocytes
- myoepithelial cells
- interdigitating reticular cells of lymph nodes
- dendritic reticular cells of lymphatic follicles
- Langerhans cells
S100 proteins are mainly expressed in the following malignancies:
- lesions arising from melanocytes incl. malignant melanoma
- lipomas and liposarcomas
- tumors from skin adnexa
- breast tumors
- salivary gland tumors
- nerve sheath tumors - staining is positive in about 50% of tumors
- carcinoids
The diagnostic value of the S100 protein is reduced precisely by its very broad expression.
- in melanoma metastases it is expressed more often than HMB-45
- makes it possible to distinguish tumors arising from cartilage from other bone tumors
- differential diagnosis of sclerosing adenosis from breast cancer (evidence of myoepithelial cells that are absent in cancer)
- differential diagnosis of Paget's disease from superficial spreading melanoma
Links[edit | edit source]
External links[edit | edit source]
Reference[edit | edit source]
- ↑ Wikipedia. https://en.wikipedia.org/wiki/S100_protein [online]. [cit. 2015-12-05]. <https://en.wikipedia.org/wiki/S100_protein>. }
Literature[edit | edit source]
- HEIZMANN, C. W. – FRITZ, G. – SCHÄFER, B. W.. S100 proteins: structure, functions and pathology. Front Biosci.. 2002, vol. 7, p. d1356-68, ISSN 1093-9946.
- LECLERC, E. – FRITZ, G. – VETTER, S. W.. , et al. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta.. 2009, vol. 1793, no. 6, p. 993-1007, ISSN 0006-3002.
- BISHOP, P.W.. An immunohistochemical vade mecum : S-100 [online]. [cit. 4/2014]. <http://e-immunohistochemistry.info/web/Antigens/s-100.htm>.
Category:Biochemistry Category:Biology https://www.wikiskripta.eu/w/Port%C3%A1l:Ot%C3%A1zky_z_patobiochemie_3_%C3%9ALBLD_%281._LF_UK,_VL%29