Reoxidative and reperfusion tissue damage
Damage to ischemic tissue after blood flow is restored. It is usually significant after a short period of ischemia. If the tissue is completely damaged, reoxygenation does not have much of an effect on it. However toxic substances ROS, NO, eicosanoids) are flushed out of dead tissue into the circulation.
Tissue damage toxins are caused by MODS – multiorgan dysfunction syndrome and SIRS - systemic inflammatory responce syndrom.
Ischemic-reperfusion injury[edit | edit source]
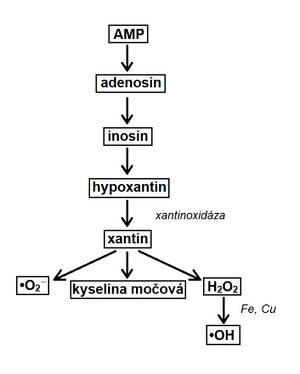
Lack of oxygen causes the transition to anaerobic glycolysis . Decreased ATP production conditions the formation of ATP from two ADP molecules. This results in ATP and AMP. After reperfusion, AMP is removed from the body by the xanthine oxidase route.
The enzyme xantin oxidaseis required to convert hypoxanthine . In humans, it is present only in the liver, intestine and lactating mammary glands .Hypoxanthine cannot be broken down in the heart. During the transformation, reactive forms, are formed , which can damage the organism (if they are not degraded fast enough).
Conversion of AMP to uric acid see scheme.
After reperfusion, ROS also form in leukocytes (no-flow phenomenon). Leukocytes adhere to each other and subsequently clog the capillaries. They also arise in mitochondria, where there is a greater escape of electrons from complexes in the respiratory chain and as a result of MPT.
MPT (Mitochondrial permeability transition)[edit | edit source]
A megacanal in the inner mitochondrial membrane that is permeable to all molecules less than 1500 daltons. Its opening is conditioned by a certain amount of calcium in the mitochondrial matrix. It can also be stimulated by oxidants, depolarizations a inorganic phosphate.Protons, [[Mg2+]], ATP, ADP and cyclosporin Acan inhibit MPT opening.
Physiologically, MPT serves for a beneficial calcium efflux from the mitochondria (calcium signaling). Pathologically , it induces cell death (apoptosis, nekrosis), or marks old mitochondria for autophagy. Opening the MPT will collapse the inner membrane potential and then balance the proton gradient. Respiration is inhibited. This is followed by swelling of the mitochondria, the release of cytochrome c into the cytosol, leading to apoptosis of the cell.
The reduced pH in ischemia protects against MPT.
During myocardial reperfusion, the conditions for MPT occur by the interplay of the following events:
- overproduction of oxidants;
- adenine nucleotide depletion;
- high concentration of P i (from ATP degradation);
- high concentration of Ca2+;
- normalization pH.
With a single MPT, H + may go out, cleaving ATP. This lowers the pH, which inhibits the formation of MPT. During reperfusion, further ATP losses occur, which worsens the condition of the cell.
Poly(ADP-ribose)-polymerase (PARP-1)[edit | edit source]
An enzyme bound to chromatin, which is activated by oxidative DNA damage. Its function is the cleavage of NAD+ and the transfer of poly (ADP-ribose) to nuclear proteins. It physiologically helps repair DNA and promotes cell survival. Excessive DNA damage causes NAD+, která urychlí buněčnou smrt. Depleci NAD+ depletion , which accelerates cell death. To some extent, NAD + depletion is also caused by MPT.
Prevention[edit | edit source]
It is still not clear. Theoretically, controlled hypothermia, MPT inhibitors (eg. Cyclosporin A), antioxidants and PARP-1 inhibitors should be prevented.
Links[edit | edit source]
[edit | edit source]
- Metabolic changes in the cell during anoxia and ischemia
- Calcium
- Mitochondria
- Ischemia
- Respiratory chain
- Glycolysis
Source[edit | edit source]
- PLÁTENÍK, Jan. Heart and nerve cell death: Ischemic-reperfusion injury. Excitotoxicity. Neurodegeneration [online]. © 2011. [feeling. 16.12.2018].<