Metabolic changes in the cell during anoxia and ischemia
Anoxia is a condition where the tissues do not have a sufficient supply of oxygen. Ischemia is a condition in which a small amount of blood flows into the tissue, or does not bleed at all. Both ischemia and anoxia are associated with insufficient oxygen supply to the tissues. Ischemic disorders are among the most common causes of death (CHD, CMP). Decreased oxygen supply leads to impaired oxidative phosphorylation in mitochondria and loss of ATP production.
Metabolic changes[edit | edit source]
In hypoxia, energy gain is converted by glycogen degradation and anaerobic glycolysis. Pyruvate is converted to lactate. Accumulation of lactate causes a decrease in pH and acidosis develops.
Anaerobic glycolysis has a much lower energy yield than aerobic, leading to a decrease in ATP (lack of energy for cells). The cell is capable of producing ATP from two ADP molecules by adenylate kinase. As a by-product, AMP is formed, which is degraded to hypoxanthine. Some cells (cardiac, nerve) are no longer able to metabolize hypoxanthine and accumulate in them.
ADP + ADP → ATP + AMP
Ionic disruption[edit | edit source]
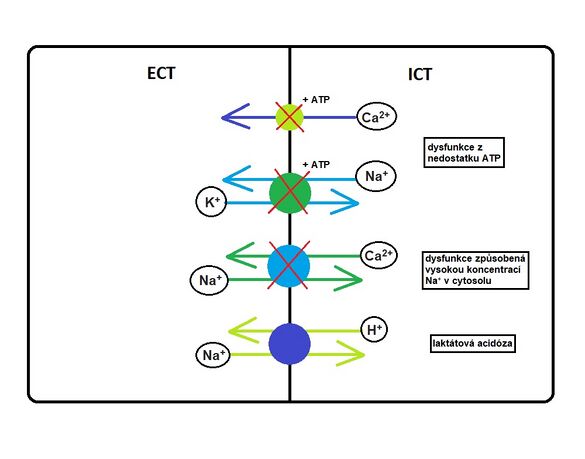
Lack of ATP prevents Na + / K + -ATPase from functioning properly. This leads to ionic disruption. Potassium exits the cell through still-open channels (leak channels). Sodium is exchanged for protons on the cell membrane, which increases its concentration intracellularly (including water). This causes intracellular edema and a decrease in pH extracellularly.
Increased intracellular calcium[edit | edit source]
Under normal circumstances, a calcium ATPase pump is involved in maintaining the calcium concentration, which maintains a very low Ca 2+ concentration intracellularly (0.1 μmol) and a relatively high Ca 2+ concentration extracellularly (2.5 mmol).
Intracellular calcium concentrations above 1 μmol cause a signal. Calcium can pass into the cytosol from the external environment (ligand-activated Ca 2+ channels, voltage-gated Ca 2+ channels) or from stores in the endoplasmic reticulum (PI3 receptor / channel, ryanodine receptor / channel).
- Physiological function of calcium
Keeping the Ca2 + concentration in the cell low is extremely important, as calcium acts as a second messenger in many important biochemical processes.
Activation of a number of enzymes:
- phospholipases (membrane phospholipid damage);
- Ca / calmodulin-dependent protein kinases;
- endonucleases (DNA fragmentation);
- endothelial and neuronal NO-synthases (eNOS, nNOS);
- some phosphatases;
- calpains (Ca-activated non-lysosomal proteases).
It is also involved in muscle contraction, release of hormones and neurotransmitters, regulation of gene expression, cytoskeletal composition and processes in the mitochondria (regulation of respiration, induction of MPT).
Mitochondrial damage[edit | edit source]
Calcium transport to mitochondria is ensured by the Ca2 + uniporter. Facilitated diffusion occurs due to the proton pump and the different charge on the membrane. Inside the mitochondria, it stimulates mitochondrial enzymes (pyruvate dehydrogenase, isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase).
The mitochondria help the cell to sequester excess cytoplasmic calcium. Due to hypoxia, the threshold intramitochondrial Ca2 + concentration is exceeded, which leads to the opening of the so-called MPT.
- MPT (mitochondrial permeability transition pore)
A megacanal in the inner mitochondrial membrane that is permeable to all molecules less than 1500 daltons. Its opening is conditioned by a certain amount of calcium in the mitochondrial matrix. It can also be stimulated by oxidants, depolarizations and inorganic phosphate. Protons, Mg2 +, ATP, ADP and cyclosporin A can inhibit MPT opening.
Physiologically, MPT serves for a beneficial calcium efflux from the mitochondria (calcium signaling). Pathologically, it induces cell death (apoptosis, necrosis), or marks old mitochondria for autophagy. Opening the MPT will collapse the inner membrane potential and then balance the proton gradient. Respiration is inhibited. This is followed by swelling of the mitochondria, release of cytochrome c into the cytosol and subsequent apoptosis of the cell.
The reduced pH in ischemia protects against MPT. The problem occurs during reperfusion.
Links[edit | edit source]
Source[edit | edit source]
- PLÁTENÍK, Jan. Smrt srdeční a nervové buňky: Ischemicko-reperfusní poškození. Excitotoxicita. Neurodegenerace [online]. ©2011. [cit. 16.12.2018]. <https://ulbld.lf1.cuni.cz/file/273/Smrt%20srde%C4%8Dn%C3%AD%20a%20nervov%C3%A9%20bu%C5%88ky%20Ischemicko-reperfusn%C3%AD%20po%C5%A1kozen%C3%AD.%20Excitotoxicita.%20Neurodegenerace.pdf>.