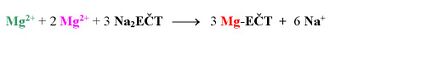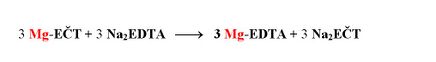Ions in drinking water
Composition of drinking water[edit | edit source]
Water found in nature contains a mixture of dissolved salts and compounds. Drinking water is water whose physical-chemical properties do not pose a threat to health. Indicators of health safety and purity of drinking water are specified in detail in Decree of the Ministry of Health of the Czech Republic No. 252/2004 Coll. The value of the drinking water quality indicator, the exceeding of which usually does not represent an acute health risk, is referred to as the limit value. The highest limit value means the value of a health-significant indicator of the quality of drinking water, as a result of which it is exceeded, the use of the water as drinking water is excluded.
Mineral waters contain more than 1 g of dissolved salts in 1 liter. Waters with a high Ca2+ or Mg2+ content are earthy (e.g. "Rudolfův pramen", "Hanácká kyselka", which contain calcium bicarbonate, "Magnesia" containing magnesium bicarbonate) or e.g. bitter ("Šaratice" containing magnesium sulfate).
A number of ions present in water are very important for the human organism, but some are undesirable and toxic at higher concentrations. Of the ions, e.g. Ca2+ ions (alone or together with Mg2+ ions), Fe3+, NH4+, NO2− and NO3− ions are monitored in drinking water.
Calcium and magnesium ions (hardness of water)[edit | edit source]
The concentration of calcium and magnesium salts is one of the indicators of water quality and is referred to as the so-called water hardness. Ca2+ and Mg2+ cations are very important for the human organism, so their presence is desirable. A high amount of Ca2+ and Mg2+ cations worsens some of the useful properties of water - settling of insoluble residues (so-called scale) on the walls of containers, reducing the effectiveness of soaps, etc.
From a health point of view, recommended concentrations in drinking water:
- Ca2+ 40–80 mg/l
- Mg2+ 20–30 mg/l
According to the amount of Ca2+ and Mg2+ salts, we distinguish:
- Transient water hardness
- it is made up of calcium and magnesium carbonates and bicarbonates. At a given temperature and pressure, there is an equilibrium between dissolved carbon dioxide (CO2) and bicarbonates (HCO3–) in the solution. By heating the solution, carbon dioxide (CO2) escapes from it, breaking the equilibrium, which leads to the gradual conversion of bicarbonate into carbonate (CO32-). Slightly soluble calcium carbonate (CaCO3) precipitates on the walls of the container or on the surface of the heating elements in the form of so-called scale.
- Permanent water hardness
- it consists of dissolved calcium and magnesium salts, in addition to carbonates and bicarbonates, i.e. sulfates, chlorides, nitrates and silicates.
- Total water hardness
- is the sum of permanent and transient hardness of water.
*Total water hardness is also given in German degrees of hardness °dH, °N. Definition of the °dH unit: 1 °dH ≈ 10 mg/l CaO or MgO. Conversion of water hardness from °dH to mmol/l: 1 mmol/l (Ca2+ + Mg2+) ≈ 5.6 °dH; 1 °dH ≈ 0.18 mmol/l (Ca2+ + Mg2+).Marking of water according to degrees of hardness* Concentration Ca2+ + Mg2+ (mmol/l) Example Very soft water < 0.5 rainwater Soft water 0.7–1.25 water from insoluble subsoils Moderately hard water 1.26–2.5 tap water Hard water 2.6–3.75 well water Very hard water > 3.8 water from limestone areas
Determination of the total content of calcium and magnesium ions in drinking water[edit | edit source]
The principle of determination is chelatometric titration - one of the methods of volumetric analysis. During titration, the cations present in the solution form complexes with some aminopolycarboxylic acids, which, although soluble, are very little dissociated. The titrant is most often a solution of the disodium salt of ethylenediaminetetraacetic acid, abbreviated Na2EDTA (chelaton 3, complexon III). Na2EDTA forms chelate complexes with polyvalent cations in which the ratio of metal and EDTA is always 1:1.
In complexometry, so-called metallochromic indicators – coloered substances that also form complexes with metal ions – are used to indicate the equivalence point. The metallochromic indicator for the determination of Mg2+ in an alkaline environment is eriochrome black T (ECT). The eriochrome black t solution is colored blue under titration conditions (pH 11). The chelate complex of eriochrome black T with Mg2+ (ECT–Mg) is colored wine red.
When determining the concentration of the magnesium ions themselves, the fact that the ECT-Mg complex is less stable than the Mg-EDTA chelate is used.
In the presence of eriochrome black T, at the equivalence point, the burgundy colour of the ECT-Mg complex changes to the blue colour of the ECT indicator itself.
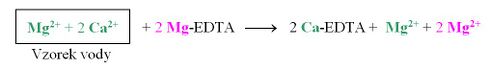
The simultaneous determination of Ca2+ and Mg2+ is then based on the fact that the Ca-EDTA chelate is more stable than the Mg-EDTA chelate. At the beginning of the titration, the Mg-EDTA chelate solution is added to the water sample solution. If calcium and magnesium ions are present in the solution in a ratio of e.g. 2:1, the reaction will occur:
This replaces all the Ca2+ cations in the solution with Mg2+ cations, which form a wine-red complex with the added indicator:
The following chelatometric titration will take place only with Mg2+, which represent the sum of Ca2+ and Mg2+ in the original sample. The end of the titration is indicated by a pure blue colour of the indicator.
Assignment: Determination of the total content of calcium and magnesium ions in drinking water - pdf.
Nitrates[edit | edit source]
Nitrates are not toxic by themselves, but they are partially reduced to toxic nitrites by the microflora of the oral cavity, and in some infections also by the intestinal microflora. This fact can be significant when a large amount of nitrates is ingested.
An acceptable daily intake is 4–5 mg NO3−/kg of body weight, while the share of NO3− intake through drinking represents an average of one third.
The highest limit value of NO3− in drinking water is 50 mg/l.
In order to meet the drinking water quality conditions, the following condition must be met:
From the point of view of prevention of nitrate alimentary methemoglobinemia, water for infants can only contain up to 15 mg NO3−/l.
In food, the highest content of nitrates is in some types of vegetables (especially root vegetables), where it often exceeds the value of 1000 mg/kg. Fruit vegetables contain the least nitrates, beetroot, greenhouse radishes and salads the most. A high concentration of NO3− in a water source usually signals the penetration of water through layers with a significant level of biological processes, and therefore a significant probability of bacterial contamination.
Proof of nitrates in water by means of diphenylamine[edit | edit source]
Nitrates oxidize diphenylamine in a concentrated H2SO4 environment to a blue colored product.
The same reaction is also provided by nitrites (even in a diluted H2SO4 environment), but these can be demonstrated by a specific diazotization reaction.
Assignment: Proof of nitrates in water using diphenylamine - pdf.
Determination of nitrates in water using salicylic acid[edit | edit source]
NO3− ions react in a strongly acidic environment with salicylic acid. Salicylic acid is nitrated and upon alkalization yields a yellow nitrosalicylate, which is determined spectrophotometrically at 410 nm.
Assignment: Determination of nitrates in water using salicylic acid - pdf.
Indicative determination of nitrates in water using Nitrotest strips[edit | edit source]
Nitrates are reduced to nitrites using a reducing agent contained in the indicator zone of the strip. Nitrous acid, which diazotizes the aromatic amine, is displaced from the nitrites by a strongly acidic buffer. Its coupling with N-(1-naphthyl)-ethylenediamine produces a red-violet colored azo compound. The intensity of the coloration of the zone is proportional to the concentration of nitrates present in the sample.
If, in addition to nitrates, nitrites are present in the sample, the colouring of the zone corresponds to their sum.
Assignment: Indicative determination of nitrates in water using Nitrotest strips - pdf.
Determination of nitrate in water using an ion-selective electrode[edit | edit source]
The electromotive voltage E of an unloaded galvanic cell consisting of a nitrate ion selective electrode (ISE) and a reference silver chloride electrode with a double salt bridge with a K2SO4 solution as a bridge electrolyte is measured (SO42− ions do not affect the potential of the nitrate ISE). The measuring part of the nitrate ISE is a solid plastic membrane in which an ionophore sensitive to NO3− ions is dissolved as a softener. The ISE changes its electric potential according to the Nernst relation, i.e. proportional to the logarithm of the activity of nitrate ions aNO3− in the solution in the range of 10−6–10−1 mol/l:
- E = const. − 59,2×log(aNO3− + selectivity coeff. × ainterfering ions)
For sufficient measurement accuracy, the following must apply:
- Activity of NO3− >> (selectivity coeff. × activity interfering ions).
If the above condition is not met, the interfering ions must be removed, e.g. by precipitation or masking in the complex.
Interfering ions for nitrate ISE in the order from max. to min.: ClO4− >> I− > Br− >> HCO3− > NO2− > Cl− >> H2PO4−, SO42−.
The membrane is very sensitive to lipophilic substances that irreversibly damage the membrane. To determine the nitrate concentration, a calibration graph is made: the measured voltage E of several calibration solutions with a known concentration of NO3− ions is plotted as a dependence of the voltage E on the logarithm of the NO3− concentration. From the measured voltage E in the unknown sample, the value of log c(NO3−) can then be subtracted from the graph and c(NO3−) can be obtained in units of mol/l by taking the logarithm.
Nitrate content in vegetable juices or extracts can be determined in a similar way.
Assignment: Determination of nitrates in water using an ion-selective electrode - pdf.
Nitrites[edit | edit source]
Nitrites are toxic (from a few tens of milligrams for humans), cause, among other things, the oxidation of hemoglobin to hemiglobin (methemoglobin) or react in the digestive tract with secondary amines, or amides taken with food to form nitrosamines, or nitrosamides, some of which are strongly carcinogenic. The formation of nitrosamines/nitrosamides is strongly suppressed with the simultaneous administration of vitamin C.
According to the decree, the highest limit value of NO2− in drinking water is 0.5 mg/l.
The presence of nitrites in water usually means significant water pollution when it passes through highly biologically active layers.
Nitrite confirmation[edit | edit source]
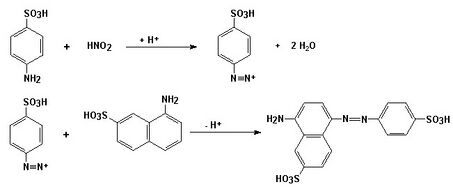
A specific and very sensitive reaction for the evidence of nitrites is the diazotization reaction, in which nitrite reacts with sulfanilic acid in an acetic acid environment to form a diazonium salt that couples with 1-naphthylamine-7-sulfonate to form a red-violet azo dye:
The method can also be used for quantitative photometric determination of nitrites.
Assignment: Evidence of nitrites by diazotization reaction - pdf.
Ammonium ions[edit | edit source]
The highest limit concentration of NH4+ in drinking water is 0.5 mg/l.
The NH4+/NH3 ratio in the solution depends on the pH value.
The presence of NH4+ cations (or ammonia in alkaline waters) is usually an indicator of gross pollution of drinking water by decomposition products of nitrogenous organic substances, mainly proteins and urea (leakage from sewers, cesspools, silage pits, etc.).
Evidence of ammonium ions[edit | edit source]
Nessler's reagent (alkaline solution K2[HgI4]) can be used to prove NH4+ ions.
The reaction is also used for the photometric determination of ammonia and ammonium salts.
Assignment: Evidence of ammonium ions - pdf.
Determination of ammonia by back titration[edit | edit source]
Ammonia is a volatile substance and significant losses occur during the titration. Therefore, back titration is used in the determination of ammonia. The essence of the back titration is to add an excess of HCl solution, which reacts with ammonia to form NH4Cl.
- NH3 + HCl → NH4Cl
The excess of the HCl solution is subsequently titrated with a standard NaOH solution.
Assignment: Determination of ammonia by back titration - pdf.
Anions of phosphoric acid[edit | edit source]
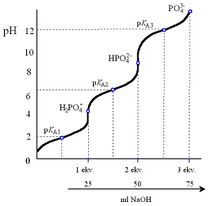
Trihydrogenphosphoric acid is a triacid (pKA1 = 2,1; pKA2 = 7,2; pKA3 = 12,3). It is stable, has no oxidising properties.
The pKA values show that it dissociates as a moderately strong acid to the 1st degree, as a weak acid to the 2nd degree, and as a very weak acid to the 3rd degree.
Verification of the solubility of phosphates depending on the pH of the solution[edit | edit source]
In phosphate solutions, depending on the pH, H2PO4− ions exist only in acidic and neutral reactions, HPO42− ions in slightly acidic to alkaline solutions, and PO43− ions only in strongly alkaline solutions. By acidifying the solutions, phosphates change to hydrogen phosphates and dihydrogen phosphates, by alkalising, the equilibrium state shifts to phosphates.
Changes taking place in phosphate solutions depending on pH are described by the phosphoric acid titration curve.
Assignment: Verification of the solubility of phosphates depending on the pH of the solution - pdf.
Carbonic acid anions[edit | edit source]
Carbonic acid is a very unstable, weak acid (pKA1' = 6,4, pKA2' = 10,3).
It can be completely expelled from the solution by heating in the form of CO2.
Evidence of carbonates and bicarbonates in solution[edit | edit source]
Assignment: Evidence of carbonates and bicarbonates in solution - pdf.
Links[edit | edit source]
Related articles[edit | edit source]
Bibliography[edit | edit source]
- BAHRUDDIN, Saad – FEN, Wei Pok. , et al. Analysis of anions and cations in drinking water samples by Capillary Ion Analysis. Food Chemistry. January 1998, vol. 61, p. 249-254, ISSN 0308-8146. DOI: 10.1016/S0308-8146(97)00024-1.
- PURI, Avinash. A review of permissible limits of drinking water. Indian J Occup Environ Med.. 2012, vol. 16, p. 40-44, DOI: 10.4103/0019-5278.99696.
- LI, Xiaoping. , et al. Major ions in drinking and surface waters from five cities in arid and semi-arid areas, NW China: spatial occurrence, water chemistry, and potential anthropogenic inputs. Environ Sci Pollut Res Int.. 2020, vol. 27, p. 5456-5468, DOI: 10.1007/s11356-019-07149-9.
- United States Geological Survey. Ground-Water Quality [online]. United States Geological Survey, [cit. 2022-12-01]. <https://pubs.usgs.gov/wri/wri024045/htms/report2.htm>.