Gamma knife
The Lexell gamma knife (LGN) is an integrated system used in stereotactic radiosurgery. It is based on focused gamma radiation from a large number of radioactive sources. It is a registered trademark of the Swedish company Elekta Instruments AB. There are currently five LGN models available - model U (original), model B, model C, model 4C and Perfexion.
Physical principles[edit | edit source]
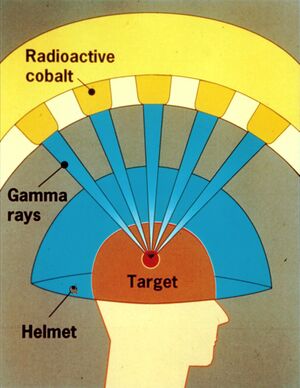
In LGN, photons are used, which are formed during the radioactive conversion of the radionuclide Co60 to Ni60. The energy of photons is high - the average energy is 1.25 MeV. Because photons do not carry a charge, there is an exponential reduction in the number of photons in a monoenergetic beam with increasing depth of penetration into a given material. Thus, the dose first increases from the surface to its maximum value, which is characterized by a certain depth for a given photon beam energy. Thereafter, the dose decreases exponentially with increasing depth.
It follows from this paragraph that the course of the beam is disadvantageous for stereotactic radiosurgery and therefore it is necessary to use a large number of collimated isocentric beams, which intersect at a single point, the isocentre. By point is meant a volume with a high dose concentration, and its size is approximately equal to the size of the collimator used. In this way, we achieve the maximum absorbed dose while the dose drops sharply into the environment.
Components[edit | edit source]
The Lexell gamma knife consists of three main components:
- a radiation unit with four collimating helmets and a treatment bed;
- Leksell's stereotactic instrumentation;
- planning system called Leksell's GammaPlan.
The radiation unit uses 201 Co60 sources , which are fixed in a hemispherical central unit with a radius of 400 mm. The sources are not placed arbitrarily but in five rows around the perimeter of the unit.
Each source contains 12-20 cylindrical pellets with a diameter and length of 1 mm, which are enclosed in two stainless steel housings. The housings are then placed in an aluminum holder. All radiation is directed (collimated) by three collimators - two are located in the radiation unit and one is in a replaceable collimation helmet. The collimating helmet in the irradiation position creates collimating openings (a total of 201) forming channels whose axis points to the focus in the center of the radiation unit with an accuracy of 0.3 mm. The collimation channel is 217.5 mm long and can be replaced by a plug that shields the beam. This is used to blind a beam of radiation that passes through a critical structure, or to blind multiple collimators to achieve an ideal spatial dose distribution.
As a stereotactic instrument, a stereotactic frameis used , which is fixed to the patient's head by means of four screws, which are firmly attached to the skull bone. The screws are made of aluminum and finished with titanium tips. The stereotactic frame is made of non-magnetic alloys of aluminum and titanium.
The Leksell GammaPlan planning system is operated on a computer with the LINUX operating system. The data is stored in a database and is accessible via passwords, which allows consultation with another workplace.
No special irradiation room is required for work with LGN because no primary beam is output outside the shielding body.
Procedure for admitting a patient[edit | edit source]
- After finding an intracranial disease that would be appropriate to treat with a gamma knife, a short hospitalization follows.
- Upon admission, the patient is instructed by a physician on the operation and treatment of the gamma knife.
- In the evening and in the morning before irradiation, the patient washes his hair with an alcohol disinfectant solution.
- The patient receives sedatives at night and in the morning.
- It is necessary to remove all metal objects before the procedure.
- Local anesthesia is performed on the head and, after piercing the skin with four spikes, attach a stereotactic frame. (for more details see section Treatment process)
- The patient is examined by X-ray or magnetic resonance imaging.
- Then an irradiation plan is prepared for several hours while the patient is resting → The control computer of the whole system checks all parameters important for the correct irradiation of the patient (collimator size, number of isocenters, irradiation time, stereotactic coordinates).
- The patient is then placed on the bed of the irradiation device and communicates with the staff using a microphone and loudspeaker.
- The irradiation itself takes a few minutes. However, it can be repeated.
- The patient is then removed from the stereotactic device and sent to rest.
- Any nausea or headache will subside by the next day.
The treatment process[edit | edit source]
- stage - acquisition of diagnostic images with marked location of the treated lesion using imaging methods such as computed tomography (CT), nuclear magnetic resonance (MR), angiography (AG) or positron emission tomography (PET).
- stage - determination of the dose distribution in the lesion and its surroundings, performed in a computational matrix.
- stage - determining the dimensions of the head using a plastic ball helmet, which has a center geometrically identical to the stereotactic frame. It is placed on the base of the frame and the distance from the skull to the surface of the plastic helmet is measured with a scale and the tissue thickness from the center of the frame to the surface of the head is also determined.
- stage - begins the actual healing process, by placing a stereotactic frame on the patient's head, under local anesthesia. The frame must be sufficiently fixed to prevent shifting. The frame cannot be used in patients under 2 years of age due to insufficient ossification and calf rigidity.
After the treatment is initiated, the screening door on the radiation unit opens and the patient lying on the bed enters the radiation unit. Primary stationary collimators with exchangeable collimators in the helmet are covered here. The correctness of the position is controlled by a microswitch with an accuracy of 0.1 mm. When the irradiation time interval set by the irradiation plan has elapsed, the bed returns to its initial position and the screening door is subsequently closed. In order to cover the entire volume of the target bearing, a larger number of such interventions may be required, depending on the size and shape of the bearing. Usually their number is less than 15 and usually does not exceed 30.
Indications[edit | edit source]
In general, LGN is used for processes whose boundary can be easily defined (eg it is not suitable for glioblastomas):
- The obliteration does not occur immediately, but within about 1-2 years - this is due to a combination of endothelial proliferation and thrombosis .
- Before they obliterate, there is still a risk of bleeding , so if they are already bleeding, it is better to obliterate otherwise, faster (rebleeding is more common).
- Cavernous malformations - indications and complications identical to AVM , the only difference being that rebleeding is more common in cavernous patients, but in the vast majority it is less clinically significant bleeding.
- Vestibular schwannoma - we irradiate mainly smaller tumors, the advantage is the possibility of maintaining hearing (if it has not been damaged) and the elimination of damage to the facial nerve .
- Neurinomas do not disappear by irradiation, some of them shrink (44%), some do not grow (42%), some continue to grow.
- With delay, the manifestations of the involvement of the cranial nerves (n. V , VII , VIII ) may occur .
- Meningiomas - mainly difficult to access on the base, in smaller ones, with contraindications for surgery in patients with severe conditions.
- Metastasis - it is possible to gradually irradiate several metastases in one session (they can be well centered).
- Pituitary adenoma - must be at least 5 mm from n. II to avoid eye damage .
Complications after irradiation[edit | edit source]
In connection with the gamma knife, we encounter radiation changes that are either acute or late.
- Acute - occurred from the day of irradiation to 90 days after treatment, and the severity of early changes may not correspond to the severity of late changes. For example, when irradiating near IV. The cerebral ventricles may experience nausea and vomiting, usually resolving within 48 hours.
- Late - complications that occurred 90 days after irradiation, the recommended time interval for monitoring late complications is 5 years. Late complications occur mainly in tissues and organs composed of differentiated cells with minimal ability to regenerate their cells. This will lose the function of part or all of the organ. In rapidly regenerating tissues (such as mucous membranes), cells are exchanged within hours and days.
Benefits of LGN treatment[edit | edit source]
- Minimally invasive method;
- does not affect healthy tissue;
- no special irradiation room is required for work with LGN because no primary beam is output outside the shielding body;
- without general anesthesia;
- fast recovery time;
- treatment success rate around 90%.
Links[edit | edit source]
Source[edit | edit source]
- BENEŠ, Jiří. Studijní materiály [online]. ©2007. The last revision 2009, [cit. ?]. <http://www.jirben.wz.cz/>.
References[edit | edit source]
- LIŠČÁK, Roman, et al. Radiochirurgie gama nožem : principy a neurochirurgické aplikace. 1. edition. Praha : Grada, 2009. pp. 248. ISBN 978-80-247-2350-1.
- ZEMAN, Miroslav, et al. Speciální chirurgie. 2. edition. Praha : Galén, 2004. pp. 575. ISBN 80-7262-260-9.