Celiac disease
Celiac disease (celiac sprue, gluten/gluten-sensitive enteropathy) is an immune-mediated, inflammatory systemic disease that is triggered by gluten contained in certain grains (and gluten-like prolamins in genetically susceptible individuals). Celiac disease has a diverse clinical picture and can be asymptomatic. Gastrointestinal symptoms, failure to thrive, growth failure and iron deficiency anemia predominate in children. The basis of the diagnosis is the detection of antibodies against tissue transglutaminase type 2 (anti-TG2), which plays a role in the pathogenesis of celiac disease. 90% of patients with celiac disease have HLA-DQ2 and the remaining 10% have the HLA-DQ8 haplotype. The causal therapy is a lifelong gluten-free diet.
Epidemiology[edit | edit source]
The prevalence in Europe and the USA is 3-13 cases per 1000 children under 15 years of age.
The incidence in first-degree relatives is 8-18%, in monozygotic twins it is approximately 70%.
The prevalence in the Czech Republic is approximately 1:250–300 across the entire age spectrum. Women are more often affected.
Etiopathogenesis[edit | edit source]
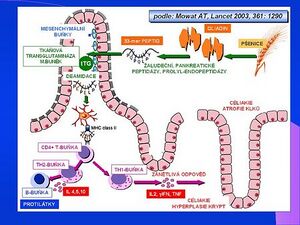
The essence of the disease is a genetically determined disorder of mucosal immunity. It reacts abnormally to gluten and prolamins found in wheat and other cereals.
Cereal grains contain a variety of proteins. These include, for example: albumins and globulins. Gluten is a group of proteins that includes glutenins and prolamins. The formation of the antibodies responsible for celiac disease is mainly caused by the structures of gliadin, the prolamin of wheat. Similar proteins from other cereals (rye or oats) can have a similar effect.
When gluten components penetrate the intestinal mucosa, they are deaminated using tissue transglutaminase. Antibodies are then formed in the lymphatic tissue of the gastrointestinal tract (GALT), which are cross-reactive to the enterocyte antigens of the intestinal mucosa.
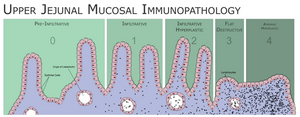
The damage to the intestinal mucosa itself occurs with the participation of T-lymphocytes. The result is mucosal atrophy with impaired absorption
Role of tissue transglutaminase[edit | edit source]
Tissue transglutaminase is an enzyme that plays a key role not only in the pathogenesis but also in the diagnosis of diseases. Gliadin and its fragments contain a high proportion of glutamine. This makes it a very good substrate for transglutaminase.
Tissue transglutaminase is located in the endomysium. It modifies a 33 amino acid long stretch of gliadin with the sequence -PQPQLPY-, which it deaminates to -PEPELPY- (ie glutamine residues are deaminated to glutamate). This creates a structure that binds to the surface glycoproteins of HLA-DQ2/DQ8 positive immunocompetent cells. This triggers an immune response and IgA and IgG antibodies begin to form against tissue transglutaminase. Tissue transglutaminase is therefore the autoantigen of celiac disease
Clinical signs[edit | edit source]
The active form of celiac sprue is characterized by a clinical manifestation of varying intensity, antibody positivity and a pathological finding on the mucosa of the small intestine upon contact with gluten. The ratio of gastrointestinal and extraintestinal symptoms is usually 1:1.
Gastrointestinal manifestations:
- recurrent abdominal pain, nausea, vomiting, flatulence, failure to thrive with weight loss, constipation.
- chronic diarrhea and failure to thrive when gluten is present in the diet (about 5% of children with celiac disease)
Extraintestinal manifestations:
- common: fatigue, osteopenia/osteoporosis, short stature, enamel hypoplasia of permanent dentition, pubertas tarda, anemia unresponsive to therapy, dermatitis herpetiformis Duhring
- less common: hepatopathy, arthritis, epilepsy with occipital calcifications.
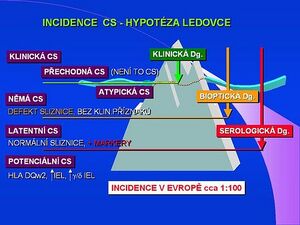
Other forms are not commonly diagnosed and account for almost 80% of cases, which are schematically divided into further groups:
- Clinically mute (silent) form of celiac disease
- It is characterized by the presence of antibodies and a typical histological picture during an enterobiopsy. Although celiac disease does not manifest itself clinically, a lifelong gluten-free diet is fully indicated.
- Latent form
- We demonstrate antibodies to tissue transglutaminase, but the mucosal architecture on enterobiopsy is normal.
- Potential form
This term sometimes refers to a population with a genetic predisposition, i.e. HLA DQw2 antigen, and an increased number of intraepithelial lymphocytes, or g/d IEL sybtypes. According to studies from 2003, the prevalence is about 1:100 in both children and the adult population.
Diagnosis[edit | edit source]
The diagnosis of celiac disease is based on anamnesis and clinical manifestations. Above all, extraintestinal forms of celiac disease can have mild and atypical manifestations, and celiac disease then appears in the diagnostic balance as a differential diagnosis to other diseases.
The diagnosis of celiac disease is based on laboratory evidence of antibodies against transglutaminase (TTG) and endomysium (EMA) of the IgA class,
IgA TTGs have a sensitivity of 96-100% and a specificity of 84-100%,
EMA IgA have a sensitivity of 88–100% and a specificity of 91–100%.
A condition for screening based on the determination of these antibodies is a normal level of total IgA immunoglobulins. In the case of total IgA deficiency, antibodies against tissue transglutaminase of the IgG class are determined.
The decisive examination for the diagnosis of celiac disease is the histological finding from a biopsy of the mucosa of the small intestine. The sample must be taken before starting the gluten-free diet. Sampling is performed using a biopsy from the duodenojejunal transition site, or during gastroscopy. Histologically, the sample is evaluated by the method according to Marsh - the morphology and number of villi and crypts of the mucous membrane and the presence of intraepithelial lymphocytes are evaluated.
The diagnosis of celiac disease is supported by the clinical and serological response to a gluten-free diet. The diagnosis of celiac disease can be considered certain if the histological findings are positive, the patient responds to the introduction of a gluten-free diet and his age is over two years.
In only a very small number of patients, celiac disease manifests itself before the second year of age. In this case, the histological finding of atrophy of the intestinal mucosa alone is not sufficiently reliable (it may be, for example, an allergy to cow's milk proteins) and it needs to be supported by further examinations.
Diseases associated with celiac disease[edit | edit source]
Celiac disease is often associated with other autoimmune diseases, especially type 1 diabetes mellitus and autoimmune thyroiditis. Patients with Down syndrome, Turner syndrome and Williams syndrome are more often affected. Some patients have selective IgA deficiency.
Treatment[edit | edit source]
The basis of the treatment is a lifelong strict gluten-free diet - complete exclusion of wheat, rye, barley and oats. Iron Patients should be monitored. As a rule, autoantibody levels are determined once a year. When following a diet, their titer decreases during the first six months. The aim of the dispensary is also the early detection of any related diseases.
Complications of untreated celiac disease[edit | edit source]
Untreated celiac disease leads to disorders caused by poor absorption of nutrients and micronutrients, but also to other disorders, the pathogenesis of which is not always clear. Disturbances of somatic development (delayed growth, puberty), osteopathy, anemia and reduced school and work performance are typical. Women suffer from fertility disorders. The risk of psychiatric diseases increases. The incidence of malignancies, especially lymphomas, is also rising significantly.
Summary video[edit | edit source]
Links[edit | edit source]
Related articles[edit | edit source]
- Screening céliakie ▪ Stanovení protilátek ke gliadinu, endomysiu nebo atTG ve stolici
- Dermatitis herpetiformis
- Bezlepková dieta
- Celiakie/kazuistika
References[edit | edit source]
- FRASER, JS, AL KING a HJ ELLIS, et al. An algorithm for family screening for coeliac disease. World J Gastroenterol [online]. 2006, vol. 12, no. 48, s. 7805-7809, dostupné také z <https://www.wjgnet.com/1007-9327/12/7805.pdf>. ISSN 1007-9327. ↑ Nekompletní citace článku. BODIS, Gergely, Victoria
- TOTH a SCHWARTING. Rheumatology and Therapy [online]. 2018, roč. 5, vol. 5, s. -, dostupné také z <https://link.springer.com/article/10.1007/s40744-018-0100-z>.
- Skočit nahoru k: FRÜHAUF, Pavel, Jiří BRONSKÝ a Petr DĚDEK, et al. Celiakie - doporučený postup pro diagnostiku a terapii u dětí a dospívajících. Pediatrie pro praxi. 2016, roč. 17, vol. 3, s. -,
- Skočit nahoru k: FRÜHAUF, Pavel. Celiakální sprue. Pediatrie pro praxi [online]. 2007, roč. 8, s. 333-335, dostupné také z <https://www.pediatriepropraxi.cz/>. ISSN 1803-5264.
- KOHOUT, P. Novinky v bezlepkové dietě. Interní medicína [online]. 2008, roč. -, vol. 3, s. 113-116, dostupné také z <http://www.solen.cz/pdfs/int/2008/03/03.pdf>.
- Tlaskalová-Hogenová H., Tučková L., Štěpánková R. et al. Imunopatogenetické mechanismy celiakie. Trendy soudobé pediatrie 1, Galén 1999, 181-197.
- Skočit nahoru k:a b c Se souhlasem autora převedeno z http://www1.lf1.cuni.cz/~kocna/
- ČSKB,. Cílený screening celiakální sprue [online]. ©2009. [cit. 2010-05-31]. <http://www.cskb.cz/cskb.php?pg=doporuceni>.
- WEST, J, et al. Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. [online]. ©2003. [cit. 2006]. <https://www.ncbi.nlm.nih.gov/pubmed/12801951?dopt=Abstract>.
- SANDERS, DS, et al. A primary care cross-sectional study of undiagnosed adult coeliac disease. [online]. ©2003. [cit. 2006]. <https://www.ncbi.nlm.nih.gov/pubmed/12655262?dopt=Abstract>.