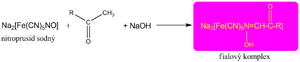Basics of organic chemistry (1.LF GM)
Alcohols and phenols[edit | edit source]
Alcohols are part of the non-aromatic hydroxy derivatives of hydrocarbons, while phenols contain a hydroxyl functional group attached directly to the benzene ring. According to the position of the –OH group in the aliphatic chain, we distinguish between primary, secondary and tertiary alcohols.
Alcohols[edit | edit source]
Primary alcohols[edit | edit source]
![]() The -OH group is attached to the primary carbon (ie, the carbon attached to only one alkyl group).
The -OH group is attached to the primary carbon (ie, the carbon attached to only one alkyl group).
Secondary alcohols[edit | edit source]
 The -OH group is attached to a secondary carbon (i.e., a carbon attached to two alkyl groups).
The -OH group is attached to a secondary carbon (i.e., a carbon attached to two alkyl groups).
Tertiary alcohols[edit | edit source]
 The -OH group is attached to a tertiary carbon (i.e., a carbon attached to three alkyl groups).
The -OH group is attached to a tertiary carbon (i.e., a carbon attached to three alkyl groups).
Examples of phenols[edit | edit source]
Phenol
The -OH group is attached directly to the benzene ring.
α-naphthol
The -OH group is attached directly to the naphthalene nucleus
For these substances a series of reactions is typical. For the purposes of practical exercises, we will focus only on the oxidation reactions of alcohols and the azo-coupling reactions of phenols.
Oxidation of alcohols[edit | edit source]
- primary alcohols are oxidized to aldehydes and then to carboxylic acids with suitable oxidizing agents
- secondary alcohols to ketones
- tertiary alcohols do not oxidize without breaking the carbon skeleton
From phenols, only compounds with two -OH groups in the o- and p- position are oxidized to form quinones.
A suitable oxidizing agent is, for example, a chrome-sulfur mixture, i.e. an orange-colored dichroman (i.e., a Cr6+ compound) with sulfuric acid, which is reduced to green chromium sulfate (Cr3+) by reaction with a primary or secondary alcohol.
An example is the oxidation of methanol to formaldehyde:
3 CH3OH + K2Cr2O7 + 4 H2SO4 → 3 HCOH + Cr2(SO4)3 + K2SO4 + 7 H2O
If we want to distinguish between primary and secondary alcohols, we will use the different qualities of their oxidation products, i.e. aldehydes and ketones. Aldehydes are reduced by Tollens', Fehling's or Benedict's reagent (see below). However, the most sensitive reaction, which does not require the production of a large amount of aldehyde and is therefore suitable for the determination of the aldehyde formed, is the reaction with Schiff's reagent.
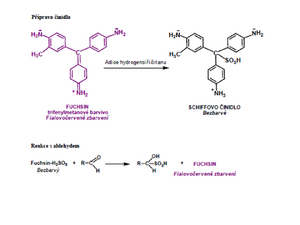
Schiff's reagent is an aqueous solution of the violet-red dye fuchsin to which bisulfite or sulfite is added. Hydrogen sulphite is adducted to the central carbon atom, thereby disrupting the quinoid structure that conditions the colour. A colorless fuchsin sulfuric acid solution is formed. After adding even a small amount of aldehyde, sulfuric acid is released from the bond to fuchsin, which binds to the aldehyde group with a stronger bond. In the fuchsin molecule, the quinoid structure is restored and the solution turns violet-red again.
The reaction is performed in the apparatus shown in the figure. The mixture of alcohol and chromic sulfuric acid is carefully heated. The resulting aldehyde/ketone is introduced into a tube with Schiff's reagent, which turns purple in the presence of aldehyde.
The reaction with Schiff's reagent is also widely used in histology in the so-called PAS reaction (Periodic Acid - Schiff) for the detection of glycogen and other polysaccharides in tissues. The principle is the oxidation of carbohydrates with periodic acid to aldehydes, which then react with Schiff's reagent to produce a purple-red coloration.
Oxidation of alcohols in the body[edit | edit source]
Ethanol is part of a number of alcoholic beverages, medicines, and is also spontaneously produced to a small extent in the digestive tract. In the body, it is metabolized mainly by the liver, a small amount of unmetabolized alcohol is excreted through the lungs, kidneys and skin. The main system of liver metabolism of ethanol is oxidation in the cytoplasm of hepatocytes. In the first step, ethanol is oxidized to acetaldehyde by the enzyme alcohol dehydrogenase (ADH, EC 1.1.1.1). The activity of this enzyme is genetically determined and may explain different individual sensitivity to alcohol. Acetaldehyde is converted by aldehyde dehydrogenases (ALDH, e.g. EC 1.2.1.3 ) to the final acetate, which is further metabolized to acetyl-CoA.
Unlike ethanol, methanol is highly toxic to the human body even in small doses. After swallowing 10 ml, irreversible damage to the optic nerve and blindness can occur, a dose of around 30 ml is fatal. The reason for the high toxicity of methanol is its oxidation using the same enzymatic systems as ethanol. ADH transforms methanol into toxic formaldehyde, from which formic acid is produced by the action of ALDH, causing hypoxia at the cellular level and metabolic acidosis. The antidote for methanol poisoning is ethanol, a competitive inhibitor of ADH, which prevents the formation of toxic metabolites and allows methanol to be excreted by the kidneys.
Azo-coupling reactions of phenols[edit | edit source]
Arendiazonium salts are formed by the reaction of aromatic amines with sodium nitrite.
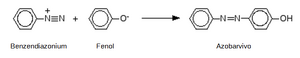
The group N+≡N is then the basis for the formation of a nitrogen bridge, which connects the arendiazonium salt with the phenolic compound in an acidic environment. The result is the formation of a colored azo compound, i.e. a substance with benzene nuclei connected by an N=N bridge.
The simplest azo-coupling reaction is the formation of an azo dye by reacting an arendiazonium salt with phenol. A yellow dye is formed, referred to as aniline yellow or Sudan yellow R.
Azo compounds are characterized by a diversely rich π electron system of bonds. If white light passes through this system, some wavelengths are absorbed by the electron system and the resulting color is actually made up of unabsorbed wavelengths. This gives rise to a whole range of colored compounds that can be used analytically in spectrophotometric determinations in biochemistry (bile dyes) or in industry in the production of dyes (diazo dyes).
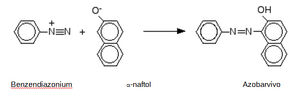
Another example of an azo-coupling reaction is the formation of an orange azo dye by an azo-coupling reaction with α-naphthol.
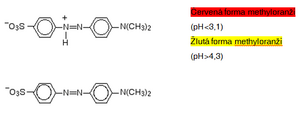
Even a relatively small change in the structure of an azo dye can result in a different absorption of wavelengths by the respective substance and thus its color that we perceive. This is used, for example, in determining pH. For example, methyl orange exists in two forms depending on pH:
Carbonyl compounds[edit | edit source]
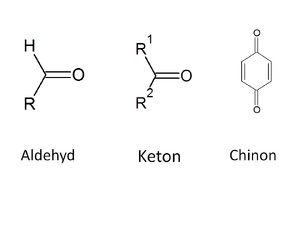
Carbonyl compounds contain a C=O group in their molecule - we call this a carbonyl or oxo group. Carbonyl compounds include aldehydes, ketones and quinones (we will not cover the last ones in this text). In the case of ketones, two carbon residues are attached to the oxo group, while aldehydes have one valence occupied by hydrogen and the other by a carbon residue (in the case of the simplest aldehyde, formaldehyde, both valences are occupied by hydrogen).
The hydrogen in the aldehyde group is a willing acceptor of electrons – the aldehyde group thus exhibits reducing properties (it itself is oxidized to a carboxyl group when the substrate is reduced). On the other hand, ketones do not have reducing properties and their oxidation occurs only with the use of strong oxidizing agents (thereby splitting the carbon chain). Due to the presence of a double bond, we are not surprised that carbonyl compounds can be the substrate of reduction reactions - they are reduced to alcohols.
Detection of aldehydes[edit | edit source]
A very sensitive method of detecting the aldehyde group is the reaction with Schiff's reagent.
Another group of reagents demonstrates the reducing feature of aldehydes. The main component is usually a metal cation, which is reduced by reaction with the aldehyde and changes color at the same time. Sometimes it is reduced to an elemental metal, which is observable as a "metallic mirror". In the reaction with Tollens' reagent, we use the reduction of silver ions to silver (black mirror), in Fehling's, Benedict's or Barfoed's exams, copper ions are reduced to reddish-brown copper oxide (possibly down to elemental copper), while in Nylander's test, metallic bismuth is formed from bismuth cations .
Carbohydrates are probably the most important aldehydes in the body, which is why the above-mentioned reactions were mainly used to determine the presence of glucose in urine. Today, more specific methods have taken over this role, so we encounter reduction tests (especially Barfoed's) mainly in the screening examination of congenital defects of carbohydrate metabolism (presence of reducing sugar in the urine).
Detection of ketones[edit | edit source]
We demonstrate the keto group by reacting with sodium nitroprusside (either in Legal's or Lestrade's exam) in an alkaline environment, when a red-violet complex is formed.
This reaction is still used today to detect ketone bodies in the urine.
Keto bodies are produced during starvation or in uncompensated patients with diabetes, when there is a lack of glucose in the cells and energy is obtained mainly by breaking down fats. Among ketone bodies, the clinic counts acetone, acetic acid and β-hydroxybutyric acid. However, it should be noted that β-hydroxybutyric acid does not belong to ketones from a chemical point of view (it has a hydroxy and a carboxy group, but not a keto group), and thus does not react with nitroprusside. A negative test result cannot rule out ketoacidosis with certainty.
Carboxylic acids[edit | edit source]
Carboxylic acids are organic compounds characterized by the presence of the -COOH group. Compared to inorganic acids, they belong to weaker acids. Their strength (i.e. the willingness to split off a proton) depends on the length of the carbon chain (the strength decreases with the length of the chain) and on any substituents (e.g. the presence of a halogen increases the strength – trichloroacetic acid is much stronger than acetic acid). Carboxylic acids (or their salts) are relatively abundant in the human body.
Important functional derivatives of carboxylic acids are esters – compounds formed by the reaction of carboxylic acid and alcohol (or phenol), which are very poorly soluble in water. Esterification takes place in an acidic environment (ideally with sulfuric acid, which will support the course of the reaction by binding the resulting water):
R-COOH + R‘-OH → R-COOR‘ + H2O
Esters of lower or aromatic carboxylic acids with lower alcohols tend to have a very distinct smell (they are often used as essences – rum, pear, pineapple, etc.). In biochemistry, however, we will mainly be interested in more complex esters - fats and waxes.
Thin layer chromatography[edit | edit source]
Thin-layer chromatography is one of the separation methods that separate substances from a mixture based on the different affinity of the individual components of the mixture to the stationary and mobile phase.
In thin-layer chromatography, a glass, aluminum or plastic plate is coated with a thin layer of a stationary phase (silica gel , aluminum oxide, etc.), onto which the analyzed sample is applied. The substance amount of the applied sample should not exceed the absorption capacity of the stationary phase (it is necessary to optimize the amount of the sample and the thickness of the stationary phase used).
Silica gel[edit | edit source]
Silica gel is a form of silicon dioxide in which silicon atoms are cross-linked to each other via oxygen molecules. Towards the surface of the wafer, –OH groups are attached to the silicon atoms. The whole structure then looks like this: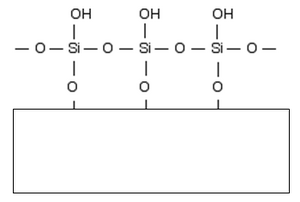
Bound –OH groups give the surface of the plate polar properties with the possibility of forming hydrogen bridges and other non-covalent interactions with separated substances.
Mobile phase[edit | edit source]
The mobile phase is a specifically defined mixture of solvents, which we pour in a small layer (up to 1 cm column of liquid) into the chromatography bath. Then we insert the chromatography plate with the applied sample into the bath so that the sample is above the surface of the solvent and that the TLC plate rests against the wall only on the back side. The mobile phase rises up the plate and carries the analyzed sample with it. The rate at which the sample is carried upward depends on the solubility of the sample in the mobile phase and the degree of interaction with the stationary phase (affinity for the stationary phase). The greater the sample's affinity for the stationary phase, the slower it rises. When the solvent arrives far enough from the start (mostly more than ¾ of the plate), we remove the plate from the chromatographic bath and mark the position on the plate where the solvent has traveled with a line (= front).
Retardation factor[edit | edit source]
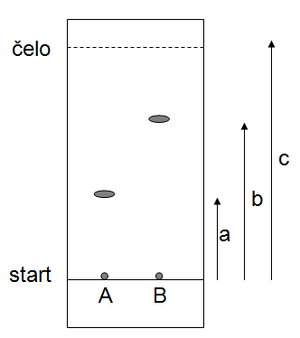
Then we measure the distance of the individual substances from the start and calculate the so-called retention (retardation) factor Rf for each substance. The Rf value indicates how far the analyte spot lags behind the solvent front. Rf is characteristic for a given substance in a given system, i.e. if we repeat the experiment, we should get the same Rf in the same arrangement. In our case, for substance A Rf=a/c, for substance B Rf=b/c (as shown at the picture).
If they are not colored substances, they must be visualized before analysis. There are several methods, from the presence of a fluorescent substance directly in the plate, when individual substances appear as black spots under a UV lamp, to chemical detection, when the developed chromatographic plate is sprayed with a reagent (ninhydrin for amino acids, etc.), which reacts with the given substances and creates such a colorful product.
Procedure:[edit | edit source]
In this hands-on exercise, we will use a plastic plate coated with silica gel. The entire procedure should be carried out due to the organic mobile phase in the digester. We do not touch the active stationary phase, we use gloves when handling the plate.
- Pour the solvent into the chromatography bath, close it with a lid and let it equilibrate for a while. Solvent vapors should saturate the internal space above the surface.
- Apply the sample to the TLC plate with a pipette or syringe and let the applied mixture dry.
- We flip the lid and insert the plate inside as quickly as possible. Close the lid immediately.
- We check that the front of the solvent does not reach beyond the upper edge of the plate. In that case, part of the sample would "go out" and it would not be possible to determine the Rf.
- We end the analysis by removing the plate from the chromatography bath and marking the front of the analysis.
Links[edit | edit source]
- LENÍČEK, – MUCHOVÁ, L.. Organika I. Návody pro praktická cvičení. 1. edition. 2011.