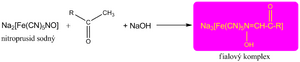Carbonyl compounds
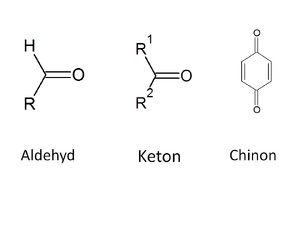
Carbonyl compounds contain a C=O group in their molecule – we call this a carbonyl or oxo group. Carbonyl compounds include aldehydes, ketones and quinones (we will not cover the latter in this text). In the case of ketones, two carbon residues are attached to the oxo group, while aldehydes have one valence occupied by hydrogen and the other by a carbon residue (in the case of the simplest aldehyde, formaldehyde, both valences are occupied by hydrogen)
The hydrogen in the aldehyde group is a willing acceptor of electrons – the aldehyde group thus exhibits reducing properties (it itself is oxidized to a carboxyl group when the substrate is reduced). On the other hand, ketones do not have reducing properties and their oxidation occurs only with the use of strong oxidizing agents (thereby splitting the carbon chain). Due to the presence of a double bond, we are not surprised that carbonyl compounds can be the substrate of reduction reactions - they are reduced to alcohols.
Evidence of aldehyes[edit | edit source]
A very sensitive method of detecting the aldehyde group is the reaction with Schiff's reagent.
Another group of reagents demonstrates the reducing properties of aldehydes. The main component is usually a metal cation, which is reduced by reaction with the aldehyde and changes color at the same time. Sometimes it is reduced to an elemental metal, which is observable as a "metallic mirror". In the reaction with Tollens' reagent we use the reduction of silver ions to silver (black mirror), in Fehlind's, Benedict's or Barfoed's tests copper ions are reduced to reddish-brown copper oxide (possibly down to elemental copper), while in Nylander's test, metallic bismuth is formed from bismuth cations.
Carbohydrates are probably the most important aldehydes in the body, which is why the above-mentioned reactions were mainly used to determine the presence of glucose in urine. Today, more specific methods have taken over this role, so we encounter reduction tests (especially Barfoed's) mainly in the screening examination of congenital defects of carbohydrate metabolism (presence of reducing sugar in the urine).
Test for ketons[edit | edit source]
We demonstrate the keto group by reacting with sodium nitroprusside (either in Legal's or Lestrade's test) in an alkaline environment, when a red-violet complex is formed.
This reaction is still used today to detect ketone bodies in the urine.
Keto bodies are produced during starvation or in uncompensated patients with diabetes, when there is a lack of glucose in the cells and energy is obtained mainly by breaking down fats. Among ketone bodies, the clinic counts acetone, acetic acid and β-hydroxybutyric acid. However, it should be noted that β-hydroxybutyric acid does not belong to ketones from a chemical point of view (it has a hydroxy and a carboxy group, but not a keto group), and thus does not react with nitroprusside. A negative test result cannot rule out ketoacidosis with certainty.
Links[edit | edit source]
Source[edit | edit source]
- LENÍČEK, M. – MUCHOVÁ, L.. Organic I. Instructions for practical exercises. 1. edition. 2011.