Aminotransferases
Aminotransferases are enzymes that catalyze transamination (the transfer of an amino group from an amino acid to a keto acid and vice versa). In clinical practice, the most important markers are alanine aminotransferase and aspartate aminotransferase, which reflect hepatocyte integrity . It follows that when the liver parenchyma is damaged, these markers will increase in the serum.
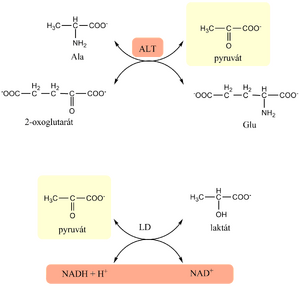
Alanine aminotransferase (ALT)[edit | edit source]
A cytoplasmic enzyme, primarily located in the liver, that is released when the cell membrane is damaged . It is a highly specific marker for liver disease. An increase in serum values occurs even with a small damage. Furthermore, it is also contained in skeletal muscles, from which it follows that an increase in serum values also occurs in myopathies.
The physiological function of ALT is to catalyze a transamination reaction in which an amino group is reversibly transferred from alanine to 2-oxoglutarate, forming pyruvate and glutamate The cofactor is pyridoxal-5´-phosphate.
| Physiological values of S-ALT | |
|---|---|
| Men | up to 0.80 μkat/l |
| Women | up to 0.60 μkat/l |
Monitoring ALT is suitable for monitoring the course of the disease.
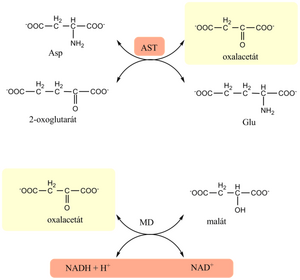
Aspartate aminotransferase (AST)[edit | edit source]
An enzyme that occurs in two forms – cytoplasmic (AST1, in the liver parenchyma about 20%) and mitochondrial (AST2, in the liver about 80%) [1]. The cytoplasmic fraction is released into the circulation relatively easily, even with mild cell damage. The mitochondrial fraction enters the blood only when the cell is necrotic . It occurs primarily in the liver, but is also found in skeletal muscle, myocardium, kidney, brain, pancreas and erythrocytes.
Its physiological function is the catalysis of reversible transamination from aspartate to 2-oxoglutarate. The cofactor of the reaction is pyridoxal-5'-phosphate.
This is a rather non-specific indicator. Very high values are found in severe liver damage (viral hepatitis, drug and toxic liver damage, acute ischemia, circulatory shock), in which ALT values are also increased. It also accompanies skeletal muscle damage, myocardial infarction, after operations, but also after prolonged physical exertion.
| Physiological values of S-AST | |
|---|---|
| Men | up to 0.85 μcat/l |
| Women | up to 0.60 μkat/l |
Determination of ALT and AST values[edit | edit source]
A method based on the principle of the Warburg optical test is recommended for determining the concentration of the catalytic activity of AST and ALT.
- Determination of ALT values
- It is based on a transamination reaction that is coupled with an indication reaction. The donor of the amino group in the transamination reaction is alanine. For the indication reaction, we use the enzyme lactate dehydrogenase (LD), which also performs the function of an enzyme ensuring the reduction of endogenous oxoacids.
- Method:
- In the first reaction catalyzed by ALT, pyruvate is formed from alanine, which is subsequently reduced to lactate in the indicative reaction catalyzed by LD, which is added to the reaction mixture together with NADH.
- The reduction of pyruvate to lactate is accompanied by a decrease in NADH, which is manifested by a decrease in absorbance at 334, 340 or 365 nm. The catalytic concentration of ALT is proportional to the decrease in absorbance.
- Determination of AST values
- Similar to ALT, it is based on a transamination reaction coupled with an indicator reaction. The donor of the amino group in the transamination reaction is aspartate, and the enzyme for the indicator reaction is malate dehydrogenase (MD).
- Method:
- In the first enzyme reaction, catalyzed by AST, oxaloacetate is formed. The latter is reduced to malate by malate dehydrogenase in the next indicative reaction, with simultaneous oxidation of NADH to NAD+.
- Based on the decrease in NADH, we will determine the activity of AST (proportional to the decrease in absorbance at 334, 340 or 365 nm).
- Pyridoxal-5'-phosphate is present in the reaction mixture, which ensures sufficient AST saturation and thus full enzyme activity. The presence of lactate dehydrogenase is necessary to ensure the reduction of endogenous pyruvate (preventing falsely higher results).
Clinical-biochemical use[edit | edit source]
Aminotransferases are part of the so-called liver tests, and are significantly used in the diagnosis of liver diseases. Liver transaminase levels are elevated in most liver diseases. The ALT value usually exceeds the AST value.
The highest values are found in acute viral hepatitis , which is why the increase in AST and ALT concentrations to several times the value is characteristic. A two- to three-fold increase in values can already be noted in the prodromal stage of the disease. The elevation peaks at 7-12. day after the onset of jaundice (maximum up to 100 μkat/l). Normalization of values usually occurs on the 5th-8th. week. Transaminases are significantly (on the order of tenfold), but only short-term, elevated in severe biliary colic. Other liver lesions are usually accompanied by a milder, maximum five-fold increase in activity, and in chronic liver diseases, transaminase activities are often just above the upper limit of the reference range.
In addition to the serum values themselves, the ratio of both enzymes AST/ALT (de Ritis index) is an important indicator for us. Values above 1 tend to be prognostically more serious. Values greater than 2 are specific for alcoholic liver damage.
In general, it can be said that the rate of increase of transaminases reflects the extent of liver damage, but no information can be derived about etiology or liver function. It should also be borne in mind that with loss of functional liver tissue , e.g. with cirrhosis of the liver, the number of cells may decrease so much that serum transaminase activities fall within the reference range or just above it even with extensive liver lesions.
Links[edit | edit source]
Related Articles[edit | edit source]
References[edit | edit source]
- ČESKA, Richard, et al. Internal 3rd edition. Prague: TRITON, 2020. 964 pp. ISBN 978-80-7553-780-5 .
Reference[edit | edit source]
- SEPULVEDA, Jorge L.. Challenges in routine clinical chemistry analysis. In Amitava Dasgupta, Jorge L. Sepulvedatitul. Accurate Results in the Clinical Laboratory . 2nd edition. Elsevier, 2019. pp. 141-163. ISBN 9780128137765