Transport of oxygen by blood
Oxygen , as a vitally important molecule, is transported by blood thanks to the blood pigment - hemoglobin . The binding of oxygen to hemoglobin has its own laws.
Oxygen bonding[edit | edit source]
Normally, 97% of the oxygen in the blood flowing from the lungs to the peripheral tissues is bound to hemoglobin (Hb). The remaining 3% are physically dissolved in the plasma . The binding of oxygen to hemoglobin is, of course, reversible. If pO 2is high, oxygen binds to hemoglobin (e.g. pulmonary capillaries), if pO 2 is low, oxygen leaves the bond with hemoglobin (e.g. tissue capillaries).
Physically dissolved oxygen occupies a volume of 3 ml in 1 liter of blood, bound to hemoglobin then around 197 ml. This is because 1 g of hemoglobin binds up to 1.34 ml of oxygen and the average concentration of hemoglobin in the blood is 150 g/l. There is therefore approximately 1 liter of oxygen in 5 liters of blood.
About 98% of the blood that enters the left atrium passes through the pulmonary capillaries and is saturated with oxygen to 104 mmHg (in our literature we often encounter a value of 100 mmHg). The remaining 2% passes through the bronchial circulation (blood gets here from the aorta) and is not in contact with the pulmonary capillaries (so-called shunt flow). This blood has a pO2 value of 40 mmHg – like normal systemic venous blood . These two components are mixed in the left atrium and thus the resulting pO2 in the left atrium is 95 mmHg .
Blood leaving the lungs therefore has a pO2 of 95 mmHg, which means that the oxygen saturation is on average 97% (see oxygen binding/dissociation curve). Blood flowing out of peripheral tissues has an average pO2 of 40 mmHg, so its saturation is 75% .
In blood that is 97% saturated, there is 19.4 ml of oxygen bound to hemoglobin per 100 ml of blood. After passing through the tissues, this value decreases to 14,4 ml O2 (pO2 40 mmHg, saturation 75%). It follows that normally 5 ml of O2 is released from the blood into the tissues for every 100 ml of blood (from 5 l of blood it is 250 ml of O2 ).
Under normal circumstances, the interstitial fluid has a pO2 of 40 mmHg (at this value, exactly 5 ml of O2 from every 100 ml of blood passes into the interstitial fluid). During vigorous exercise , this value drops to 15 mmHg. This means that more oxygen is released from hemoglobin until only 4,4 ml of it remains bound to Hb in 100 ml of blood. Thus, 15 ml of O2 reaches the tissue from every 100 ml of blood (= 19,4 – 4,4). The release of oxygen will thus increase threefold (which, with an increase in cardiac activity - six to seven times - gives up to a twenty-fold increase in oxygen transport to the tissues).
Dissolved oxygen in blood with a pO2 value of 95 mmHg is about 0,29 ml per 100 ml of blood. After passing through the tissues (40 mmHg), dissolved oxygen is 0,12 ml per 100 ml of blood. This means that only 0,17 ml of dissolved O2 from every 100 ml of blood is released into the tissues (3% of the O2 transported from the blood to the tissue - see above). During exercise, the proportion of that transport can drop to 1,5%. On the contrary, when inhaling oxygen with a high pO2 , this proportion may increase and there may even be a risk of oxygen poisoning.
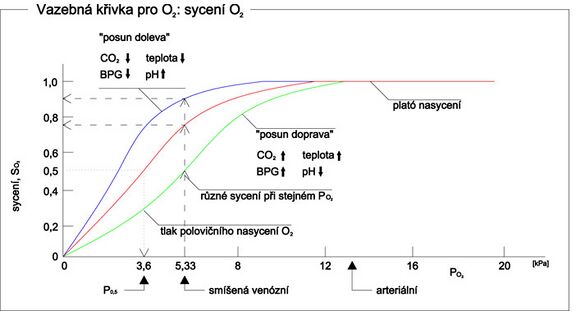
Factors affecting hemoglobin saturation[edit | edit source]
pH[edit | edit source]
- The lower the pH , the lower the affinity of hemoglobin to oxygen. Move right. (The so-called Bohr effect )
- The higher the pH, the higher the affinity of hemoglobin for oxygen. Move left.
pCO2[edit | edit source]
- An increase in pCO 2 leads to a decrease in pH. This leads to a decrease in hemoglobin's affinity for oxygen. Move right.
- A decrease in pCO 2 leads to an increase in pH. This leads to an increase in the affinity of hemoglobin for oxygen. Move left.
Temperature[edit | edit source]
- The higher the temperature, the lower the affinity of hemoglobin for oxygen. Move right.
- The lower the temperature, the higher the affinity of hemoglobin for oxygen. Move left.
2,3-Bisphosphoglycerate[edit | edit source]
- The higher the concentration of 2,3-bisphosphoglycerate (BPG), the lower the affinity of hemoglobin for oxygen. Shift of the saturation curve to the right.
- The lower the concentration of 2,3-BPG, the higher the affinity of hemoglobin for oxygen. Shift of the saturation curve to the left.
How it is in the body[edit | edit source]
- Lungs = decrease in pCO 2 → higher pH → shift of the saturation curve to the left → oxygen binds to Hb
- Tissues = increase in pCO 2 → lower pH → shift of the saturation curve to the right → oxygen is released from Hb
Measurement[edit | edit source]
Hemoglobin oxygen saturation is measured by pulse oximetry . Saturation measurement is also part of the finger plethysmograph examination .
Links[edit | edit source]
Related articles[edit | edit source]
Reference[edit | edit source]
- ↑ TROJAN, Stanislav. Medical physiology. 4., reworked and edit edition. Grada Publishing, a.s, 2003. 772 pp. ISBN 80-247-0512-5.
References[edit | edit source]
- TROJAN, Stanislav. Medical physiology. 4., reworked and edited edition. Prague : Grada Publishing, a.s, 2003. 772 pp. ISBN 80-247-0512-5.
- GUYTON, Arthur C – HALL, John E. Textbook of Medical Physiology. 11. edition. Elsevier, 2006. 11; ISBN 978-0-7216-0240-0.