Polypeptide signal sequence, free and bound ribosomes
Cytosolic proteins are synthesized on ``free cytosolic ``ribosomes, while membrane proteins, organelle proteins, and proteins released outside the cell are synthesized on ribosomes bound to the rough endoplasmic reticulum (ER). Free and bound ribosomes are structurally and functionally identical, their binding to the ER is determined by the sequence of the synthesized chain. Most proteins determined outside the cytosol have a so-called signal sequence of 13 - 16 amino acids at the N-terminus. Although these sequences vary from protein to protein, the presence of several hydrophobic amino acid residues is characteristic. The sequence is recognized by a signal recognition particle (SRP) consisting of six protein subunits and 7SL RNA. It binds to the signal sequence of the synthesized protein and stops
translation in the initial phase. The ER membrane contains receptors for SRP. As soon as the ribosome-SRP complex binds to them, proteosynthesis continues with the participation of two other membrane proteins, ``ribophorin I and II, and at the same time the peptide chain passes through the membrane into the ER cistern. SRP is again released from the receptor into the cytosol.
The signal sequence is critical for translocation. If, by genetic manipulation, it is attached to a cytosolic protein, e.g. hemoglobin, then this protein is released outside the cell. Peptide translocation is an active membrane process, requiring energy (ATP). Traversal is not driven by translation, the ribosome. Theoretically, it could take place even after the chain synthesis is completed on the free ribosome. However, early binding of the synthesized protein and ribosome to the ER is advantageous and usually necessary, because after synthesis on a free ribosome, the protein could adopt a conformation that would make translocation through the membrane impossible.
The tight space between the site of translation and translocation does not allow the chain to conform until on or within the other side of the membrane. Some proteins remain anchored in the membrane, which is also determined by their primary sequence.
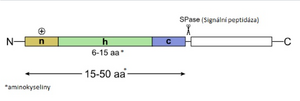
In addition to the signal sequence, membrane proteins also have an anchoring, stop-transferase sequence, which terminates translocation across the membrane and the protein remains an anchored part of the membrane. The signal sequence of these proteins can be somewhat distant from the N-terminus. Some even have several such sequences, alternating with stop-transfer sections, so that they are anchored in the membrane in several ways, sometimes several times (see figure). The signal sequence of the secreted proteins is still cleaved by the membrane signalase during translocation. The protein enters the ER cisterna. Here, and especially in the Golgi apparatus, it is covalently modified (see Post-translational glycosylation of proteins) and then transported to the place of its function.
Links[edit | edit source]
References[edit | edit source]
- {{#switch: book
|book =
Incomplete publication citation. . Concise Biochemistry : storage and expression of genetic information. Prague : Medprint, 1998. 978-80-7262-438-6.
|collection =
Incomplete citation of contribution in proceedings. . Concise Biochemistry : storage and expression of genetic information. Prague : Medprint, 1998. {{
#if: 80-902036-2-0 |978-80-7262-438-6} }
|article =
Incomplete article citation. . 1998, year 1998,
|web =
Incomplete site citation. . Medprint, ©1998.
|cd =
Incomplete carrier citation. . Medprint, ©1998.
|db =
Incomplete database citation. Medprint, ©1998.
|corporate_literature =
. Concise Biochemistry : storage and expression of genetic information. Prague : Medprint, 1998. 978-80-7262-438-6} }